Stability of bubbles in the foaming process of foam aluminum
Wei Li 1, Li Zhenjiang 2, Yao Guangchun 1
(1. School of materials and metallurgy, Northeastern University, Shenyang 110004, Liaoning; 2. Shenyang Science and technology entrepreneurship center, Shenyang 110005, Liaoning)
Abstract: the influence of bubble stability on foaming effect in the process of preparing foam aluminum materials by powder metallurgy was studied. The factors affecting bubble stability were analyzed. It was determined that the viscosity of melt and the decomposition of titanium hydride were the main factors determining bubble stability. Calcium was added to Al Si alloy powder to increase melt viscosity and control melt surface tension; The amount of hydrogen released from the decomposition of titanium hydride is controlled by controlling the amount of titanium hydride added; Control the foaming time so that the foaming can be carried out within the stable period of bubbles; Keeping the balance between the gas pressure in the bubble and the surface tension of the bubble can obtain foam aluminum materials with uniform pore structure and suitable density
Key words: powder metallurgy; Foam aluminum; Bubble; Melt viscosity; Stability; Decomposition of titanium hydride
Chinese Library Classification No.: t f 34 document identification code:
Because of its unique properties, foam has become a hot material in the forefront of research in the 21st century. In recent years, there have been extensive studies on the preparation methods, microstructure, mechanical properties and applications of foam materials at home and abroad [1,2], but most of them focus on the macro impact of the preparation process on the pore structure and properties, The research on the foaming process of foam aluminum is also mostly focused on the direct foaming method [3,4]. Although some research work has been carried out on the process parameters and preparation process of powder metallurgy preparation of foam aluminum materials [5~ 7], there are few reports on the influence of bubble stability on foaming effect in the foaming process. Because the foaming process is quite complex, The formation of foam structure is controlled by the complex interaction between bubbles and liquid interface, so improving the stability of bubbles is a very important problem in the process of preparing foam aluminum materials by powder metallurgy. This paper studies the factors affecting the stability of bubbles, uses a special way to add calcium to increase viscosity to control the stability of bubbles, and explores the control conditions of bubble stability.
1 test method
Using aluminum silicon alloy containing 125% silicon as raw material, add different amounts of calcium into the aluminum silicon alloy, and then prepare it into powder. Mix the aluminum silicon calcium alloy powder and foaming agent (TiH2) evenly, and then press it into metal preforms. The furnace temperature rises to 580. Put the preforms formed by pressing into the heating furnace, keep them warm for an appropriate time, take them out for cooling, and you can get foam aluminum materials. Calculate the density of foam aluminum by volume measurement method, and cut the sample open by wire cutting, The hole structure test device of foam aluminum photographed by digital camera is shown in Figure 1
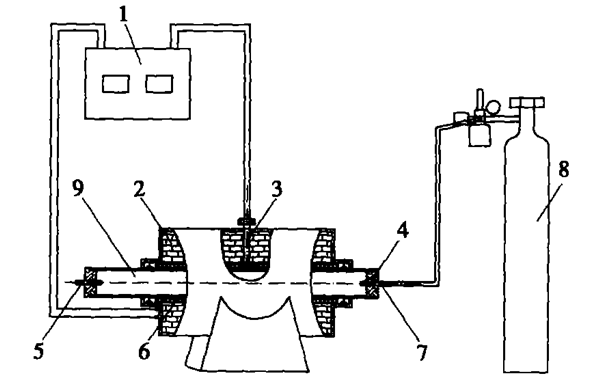
Fig. 1 Schematic of experimental setup for foaming tests
1. Temperature controller; 2. Insulation layer; 3. Thermocouple; 4. Sealing plug;
5. Exhaust pipe; 6. Silicon carbide rod; 7. Intake pipe; 8. High purity argon; 9. Furnace
2 results and discussion
Foam is formed by the dispersion of bubbles in liquid. The deformation of bubbles in liquid depends on the interaction between bubbles. Due to the formation of metal gas interface, the surface energy will inevitably be generated. The foam will always be in a non-equilibrium state, and there is a trend to reduce the internal energy by reducing the internal surface. That is to say, the porous structure of foam changes with time, and its
The actual structure depends on the technological process. Affected by the heat and force of the process, the structure of foam is generally chaotic, and the evolution of the structure depends on the joint action of three mechanisms: membrane rupture makes bubbles merge; Gas diffusion makes bubbles grow from small to large and break; Gravity forces the liquid to drain downward. For a single bubble without boundary in the melt, the pressure balance at the gas-liquid interface can be expressed by Rayleigh equation [8]:

Where P B is the bubble pressure; P L is the equilibrium pressure in the liquid; Is surface energy; R is the diameter of spherical bubble; P 0 is atmospheric pressure; G is the acceleration of gravity; H is the depth of the bubble; Is density
During the foaming process, bubbles are in a dynamic equilibrium process of growth, consolidation and collapse. The generation rate of bubbles, the size and distribution of bubbles, and the properties of liquid-gas interface are all factors that affect the stability of bubbles. Different foaming conditions, the main factors that affect the stability of bubbles, and the process characteristics of powder metallurgy determine that the hydrogen produced by the decomposition of TiH2 is
Wrapped by molten aluminum, with the increase of the amount of hydrogen in the bubble, the bubble pressure increases, and the bubble gradually grows. When the surface tension of the bubble wall aluminum melt interface is exceeded, the bubble will rupture. When the two bubbles are in contact and the surface tension of the aluminum melt is small, the bubbles will spontaneously merge. When the pressure in the bubble is balanced with the surface tension of the bubble, the bubble stability is good, and the surface tension of the bubble is determined by the viscosity of the melt, The pressure of the gas in the bubble is determined by the amount of hydrogen released by the decomposition of titanium hydride. Therefore, the viscosity of the melt and the decomposition of titanium hydride are the main factors that determine the stability of the bubble. During the foaming process, the stability of the bubble can be controlled by controlling the viscosity of the melt, the amount of foaming agent and the foaming time
2.1 calcium content
Figure 2 shows the pore structure photos of foam aluminum with different calcium content. It can be seen that when the mass fraction of calcium is 15%, the melt viscosity is small, the surface tension of the bubble liquid film is small, the bubbles are easy to grow and merge, and hydrogen is easy to escape from the melt. Therefore, there are large and interconnected holes in Figure 2A. At the same time, due to the escape of hydrogen, there is a bubble free zone in the sample. With the increase of the mass fraction of calcium (25%), the melt viscosity gradually increases, On the one hand, it can better inhibit the escape of hydrogen, on the other hand, it hinders the consolidation of holes
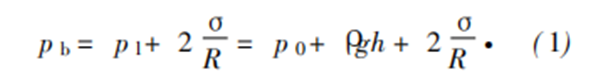
Where P B is the bubble pressure; P L is the equilibrium pressure in the liquid; Is surface energy; R is the diameter of spherical bubble; P 0 is atmospheric pressure; G is the acceleration of gravity; H is the depth of the bubble; Is density
During the foaming process, bubbles are in a dynamic equilibrium process of growth, consolidation and collapse. The generation rate of bubbles, the size and distribution of bubbles, and the properties of liquid-gas interface are all factors that affect the stability of bubbles. Different foaming conditions, the main factors that affect the stability of bubbles, and the process characteristics of powder metallurgy determine that the hydrogen produced by the decomposition of TiH2 is
Wrapped by molten aluminum, with the increase of the amount of hydrogen in the bubble, the bubble pressure increases, and the bubble gradually grows. When the surface tension of the bubble wall aluminum melt interface is exceeded, the bubble will rupture. When the two bubbles are in contact and the surface tension of the aluminum melt is small, the bubbles will spontaneously merge. When the pressure in the bubble is balanced with the surface tension of the bubble, the bubble stability is good, and the surface tension of the bubble is determined by the viscosity of the melt, The pressure of the gas in the bubble is determined by the amount of hydrogen released by the decomposition of titanium hydride. Therefore, the viscosity of the melt and the decomposition of titanium hydride are the main factors that determine the stability of the bubble. During the foaming process, the stability of the bubble can be controlled by controlling the viscosity of the melt, the amount of foaming agent and the foaming time
2.1 calcium content
Figure 2 shows the pore structure photos of foam aluminum with different calcium content. It can be seen that when the mass fraction of calcium is 15%, the melt viscosity is small, the surface tension of the bubble liquid film is small, the bubbles are easy to grow and merge, and hydrogen is easy to escape from the melt. Therefore, there are large and interconnected holes in Figure 2A. At the same time, due to the escape of hydrogen, there is a bubble free zone in the sample. With the increase of the mass fraction of calcium (25%), the melt viscosity gradually increases, On the one hand, it can better inhibit the escape of hydrogen, on the other hand, it hinders the consolidation of holes

As can be seen from Fig. 2B, there is basically no bubble free area, and there are very few interconnected holes. The holes are circular and uniform in shape. When the mass fraction of calcium is increased to 30% - 35%, the melt viscosity further increases, which increases the surface tension of the bubble liquid film, increases the thickness of the liquid film, reduces the chance of merging between bubbles, and the bubbles are more stable. It can be seen in Fig. 2C and Fig. 2D, The pores tend to be more circular, but the pore wall thickens, the number of small pores increases, and the density of foam aluminum increases. The experimental results show that adding calcium with a mass fraction of 25% - 30% can obtain better foaming effect.
2.2 addition of titanium hydride
Figure 3 shows the pore structure of foam aluminum with different amounts of titanium hydride. Figure 3A shows that the amount of TiH2 added is insufficient. A small amount of TiH2 particles are surrounded by a large number of metal particles. To achieve a certain degree of foaming, the expansion volume of hydrogen generated by TiH2 decomposition is large, so it can be observed that a small number of irregular macropores still cannot be foamed in the parts without TiH2 distribution. Therefore, it can be seen that the solid core that has not been foamed increases with the content of TiH2, TiH2 particles are uniformly surrounded by aluminum silicon alloy particles, and bubbles grow up by overcoming the resistance of the melt. In the process of growth, bubbles squeeze each other, making bubbles smaller. As shown in Fig. 3b and Fig. 3C, when the mass fraction of TiH2 is 10% and 13%, there are a large number of evenly distributed circular holes. When the mass fraction of TiH2 is 20%, as shown in Fig. 3D, there are some large and extremely irregular pores and broken holes, This is because too much TiH2 is added, and the resulting gas pressure is too large, resulting in unstable bubbles, bubble wall rupture and consolidation or hole breaking, forming large and irregular pores. It can be seen that when the mass fraction of TiH2 is 10% - 13%, the bubbles are stable, and foam aluminum materials with uniform pore diameter and appropriate density can be obtained.
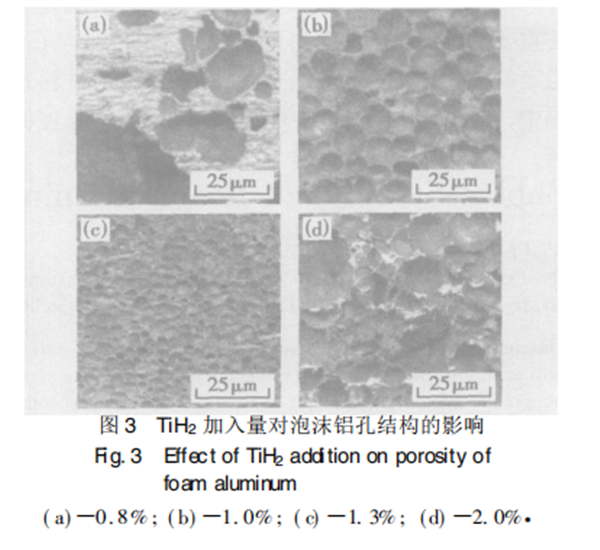
2.3 foaming time
Figure 4 shows the relationship between foaming time and foam aluminum density.
It can be seen that in the initial stage, the density of foam aluminum decreases with the increase of foaming time. After a certain period of time, it increases with the increase of foaming time. In the initial stage of foaming, the number of molecules of hydrogen released by the decomposition of TiH2 continues to increase, resulting in the increase of gas pressure in the bubble and the enhancement of foaming power. The density of foam aluminum obtained during this period continues to decrease, which is the bubble stability stage. After a certain period of time, The gas pressure in the bubble is greater than the surface tension of the bubble wall, the bubble becomes unstable, some bubbles break and collapse, and the bulk density of foam increases.
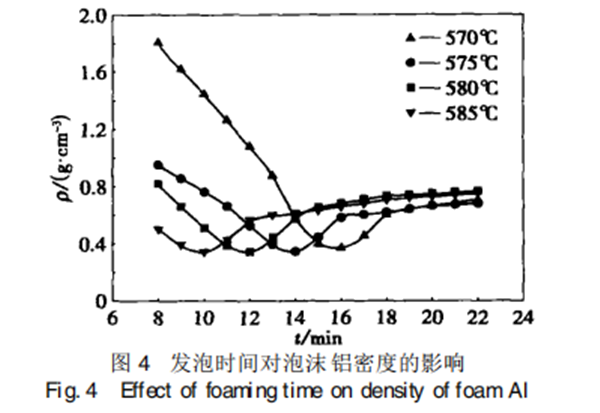
At the same time, the foaming temperature affects the speed of gas produced by the decomposition of foaming agent and the speed of gas expansion. With the increase of temperature, the decomposition rate of foaming agent increases, and the high decomposition rate leads to the acceleration of gas expansion. Therefore, the higher the foaming temperature is, the shorter the bubble stabilization period is, and the shorter the time for the aluminum density of foam to stabilize at the minimum point. The test results show that if the foaming time is controlled within 10 ~ 16 minutes, the aluminum material with lower density can be obtained.
4 Conclusion
During the foaming process, the bubble stability is of great importance to the foaming effect. The viscosity of the melt and the decomposition of titanium hydride are the main factors determining the bubble stability. In the process of preparing foam aluminum materials by powder metallurgy, adding calcium to aluminum silicon alloy powder is an important measure to improve the viscosity of the melt. The decomposition of titanium hydride has a great impact on the bubble stability. The hydrogen released by the decomposition of titanium hydride is the driving force of bubble growth, Therefore, maintaining the balance between the gas pressure in the bubble and the surface tension of the bubble is the key to obtain a foam aluminum material with uniform pore structure and suitable density. The molecular number of hydrogen released from the decomposition of titanium hydride can be controlled by controlling the amount of titanium hydride added, the foaming time can be controlled, the foaming can be carried out in the stable period of the bubble, and the surface tension of the bubble can be controlled by controlling the melt viscosity.

