Iron Boride(FeB)







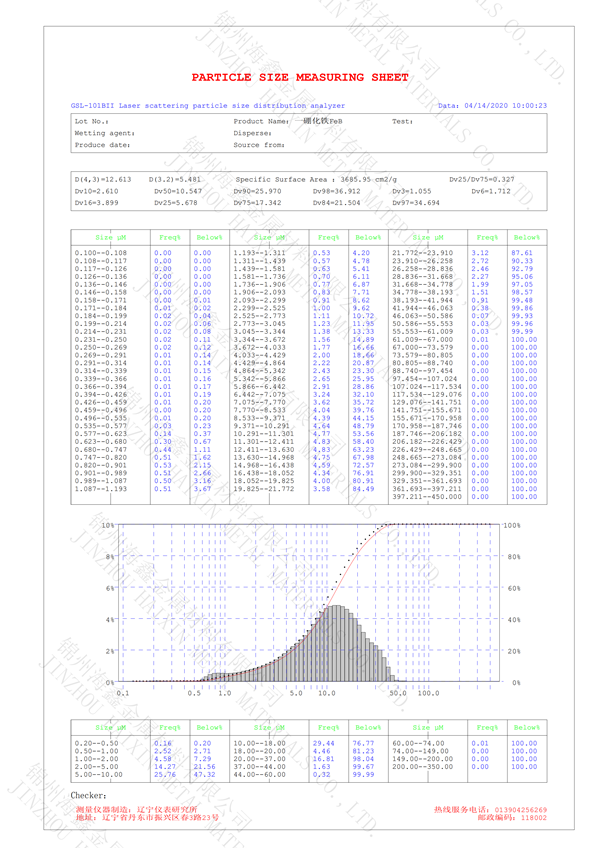

Chinese Name: Iron borate
Chemical formula: Feb
Molecular weight: 66.66
Melting point: 1652 ℃
Density: 7.15g/ml (25/4 ℃)
CAS No.: 12006-84-7
Production method:
1. Mix boron and iron in a molar ratio of 1:1, and heat them in argon at 1200 ~ 1300 ℃.
2. FES and BCl3 are reacted in hydrogen at a temperature higher than 500 ℃, or
prepared by the reaction of ferrous chloride solution with sodium borohydride (NaBH4).
Material structure:
Iron borate is arranged in a sawtooth chain shape, with iron atoms on the corners
of the triangular prism, while boron atoms are located in the center of the triangular
prism and connected in the sawtooth chain in the form of covalent bonds.
Physical and chemical properties:
The melting point of iron borate is 1652 ℃, the density is 7.15g/ml, and it is dissolved
in dilute and concentrated nitric acid, concentrated and 1:1 hydrochloric acid solution,
1:1 H2SO4 and HClO4, μ eff=1.1 μ B。 At low temperature α- Exists in the form of with
the same TC (602k) at high temperature β- Shape exists. With the change of magnetic
moment α towards β Type transformation. Both can form ferromagnets. React with
boiling water. It is hard, refractory and has good corrosion resistance.
Iron borate has ceramic properties, such as high hardness, and metal properties, such
as thermal conductivity and conductivity. Boride coating on iron surface has excellent
mechanical properties, friction properties and corrosion resistance. Ferroboron (Feb)
is a gray powder, insoluble in water. Feb is harder than Fe2B, but more brittle, and easier
to break under impact.
Purpose:
1. Target;
2. Ferroboron is a strong deoxidizer and boron additive in steelmaking production. The
biggest role of boron in steel is to significantly improve the hardenability and replace
a large number of alloy elements with only a very small amount of boron. In addition,
it can also improve the mechanical properties, cold deformation properties, welding
properties and high temperature properties.
Preparation of ultrafine iron borate:
After mixing ferrous gluconate solution with boric acid solution, spray drying is carried
out to obtain spray drying material. The molar ratio of ferrous gluconate solution to boric
acid is 1:1.01 1.02; The spray dried material was sintered in methane atmosphere for
5 hours 10h, sintering temperature 800 Reaction at 1200 ℃ to obtain the reducing
material; Continue to inject carbon dioxide into the reducing material at the temperature
of 700 850 ℃ reaction 2 4h, get sinter; Superfine iron borate is obtained by crushing,
screening and vacuum packaging the sintered material. This method can prepare ultrafine
iron borate with small particle size, large bet and high purity.