Magnesium Boride Powder(MgB2)

.jpg)

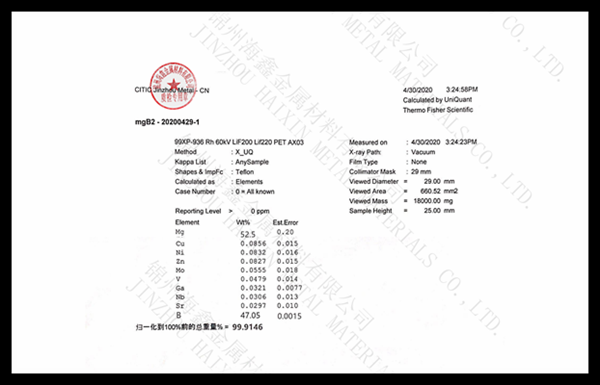
.jpg)
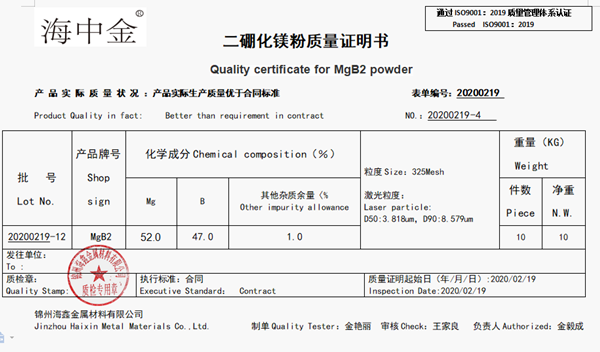

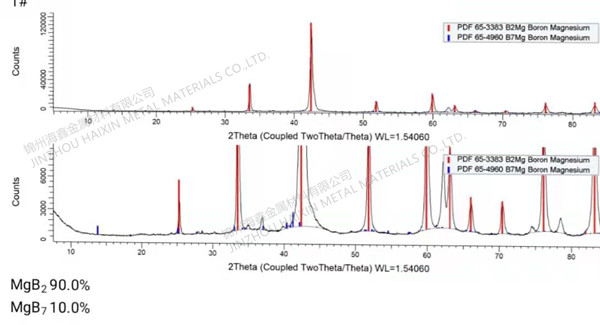
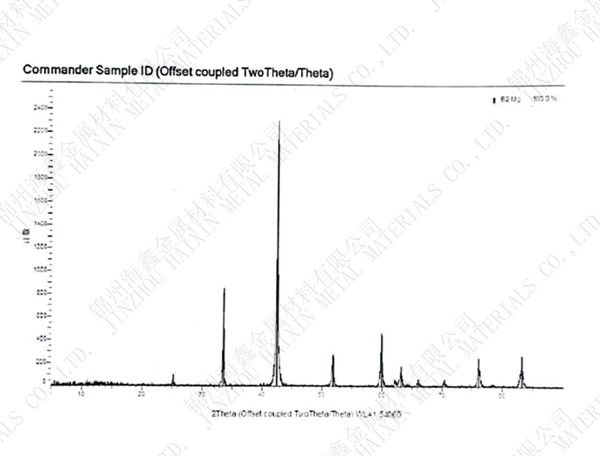
.jpg)

Magnesium diboride
Also known as magnesium borate
Chemical formula MgB2
Molecular weight 45.93
CAS number 12007-25-9
Melting point 830 ℃
The density is 2.57g/cm3
Magnesium diboride is an ionic compound with hexagonal crystal structure. It is a brittle
and hard material with poor ductility. It is an intercalation compound. Magnesium and
boron layers are arranged alternately. It will transform into superconductor at a temperature
slightly close to the absolute temperature of 40K (i.e. - 233 ℃). Its transition temperature
is almost twice as high as that of other superconductors of the same type, and its actual
working temperature is 20 ~ 30K. The superconducting transition temperature of MgB2
is 39K, that is minus 234 ℃, which is the highest critical temperature of metallic compound
superconductors. As a new material with superconductivity, magnesium diboride opens
up a new way to study the new generation of high temperature semiconductor with
simple structure. The superconductor magnesium diboride is a metal compound formed
by the combination of magnesium and boron in the ratio of 1:2. It is characterized by
abundant resources, low price, high conductivity, easy synthesis and simple processing.
Because MgB2 is easy to be made into thin films and wires, it can be widely used in the
manufacture of CT scanners and other electronic instruments, components of supercomputers
and components of power transmission equipment. It has a broad application prospect
in the field of electronics and computer. A kind of high density MgB2 superconductor
sample was successfully synthesized by high temperature and high pressure method
in China, which is close to the international level. Potential applications of MgB2 include
superconducting magnets, power transmission lines and sensitive magnetic field detectors.
In 2001, researchers found that a nondescript compound, magnesium diboride, transforms
into a superconductor at a temperature slightly close to the absolute temperature of
40K (- 233 ℃). Its transition temperature is almost twice as high as that of other superconductors
of the same type, and its actual working temperature is 20 ~ 30K. To achieve this temperature,
liquid neon, liquid hydrogen or closed cycle refrigerator can be used to cool the temperature.
Compared with the industrial cooling of niobium alloy (4K) with liquid helium, these methods
are simple and cost-effective. Once doped with carbon or other impurities, the ability of
magnesium borate to maintain superconductivity is no less than that of niobium alloy or
even better in the case of magnetic field or current. Its potential applications include
superconducting magnets, power transmission lines and sensitive magnetic field detectors.
.jpg)
.png)

Magnesium diboride is an inorganic compound with the chemical formula mgb‹. Amorphous magnesium diboride is a dark gray granular solid insoluble in water. In 2001, researchers found that magnesium diboride was transformed into a superconductor at 39 Kelvin, which is a conventional superconductor. MgB Ψ is significantly different from most conventional superconductors containing transition metals.
The discovery of magnesium diboride (MgB2) superconductivity has caused a sensation in the condensed matter physics world because it has created a new record for the transition temperature of intermetallic compound superconductors, which is as high as 39K. Different from the alloy cryogenic superconducting materials, it can work in the working temperature range of the refrigerator (20-30k), thus reducing the refrigeration cost of the expensive liquid helium temperature range. Oxide high temperature superconductors have the advantage of high superconducting transition temperature, but the coherent length is small, which leads to low cohesive energy and flux pinning energy; Layered structure leads to anisotropy; Ceramic properties make the materials easy to crack and the raw materials are more expensive, which are very limited in application. Compared with ceramic high-temperature superconducting materials, MgB2 has easy forming, weak anisotropy and large coherent length, which makes MgB2 have a good application prospect.
The measured calorific value and combustion efficiency of MgB2 are higher than those of amorphous boron In the temperature range of 298 ~ 1673 K, the thermal oxidation reaction of MgB2 under the condition of slow heating up includes four stages, and the main oxidation heat release and weight gain occur between 1200 ~ 1665 K The main oxidation heat release and weight gain of amorphous boron occurred near 1919 K At 1665 K, the oxidation rate of MgB2 is as high as 94.3%, while that of amorphous boron is only 43.6% Compared with amorphous boron, MgB2 can be fully oxidized at lower temperature, and its thermal oxidation characteristics are better than that of amorphous boron.
The superconducting principle of MgB2 is similar to that of metal. It is that electricity is connected into pairs by the quantized vibration of sound, and superconductivity is formed through materials in the form of sound waves. Therefore, MgB2 belongs to the category of BCS theory. BCS theory was developed by batting in 1957 The theory proposed by Cooper and Schriever to explain conventional superconductors Both the classical electro - acoustic coupling theory and the electron phonon coupling theory It can well explain the superconductivity of metals and intermetallic compounds. The results show that (1) the superconductivity source JB of MgB2 has the original phonon spectrum, and the superconducting current density is high, and the grain boundary relative superconducting current is "transparent ". that is, the superconducting current is not limited by the connectivity of the product boundary, and is especially suitable for strong current transmission and making high-quality microwave devices. Second, MgB2 is made of relatively cheap raw materials b.mg, and the synthesis is also simple. While the oxide high-temperature superconductor is composed of a variety of elements. The raw materials are expensive, and the materials are brittle: large, so it is difficult to be processed into practical wires. In short, the weakness of low critical temperature of MgB may be compensated by its advantages in preparation, processing and price. Table 1 shows the basic performance indexes of MgB2. Research status of superconducting properties of 3mgb2
At present, the research contents of various countries mainly focus on the factors affecting the critical temperature of MgB2 So that it can be used as soon as possible. Scientists usually use two "doping" methods to change the critical temperature of MgB2: one One is electron doping. MGB, -, x, (x= be.c.n.o, etc.) is synthesized, i.e. be C. N.O and other elements partially replace element B in MgB2. So far, it has not been found that electron doping can improve the critical temperature: the other is empty six doping. Synthetic mg M. B2 (m= al.be.Ca, Cu, ll.na.zn, etc.), i.e. Al, be Ca . Cu,Ll. Na. Zn and other elements partially replace mg. By doping or substituting, the carrier concentration of the material can be changed, thereby changing TC. Chinese scientists have found that MGB (i.e. MgO, CuO XB2) doped with 20% copper has superconductivity, and its superconducting transition starting temperature is 49k The zero resistance temperature is 45 6K. Mgocuozb2 is mainly a mixture of MgB2 and cuzmg. Its bulk structure is still hexagonal, but its c-axis and a-axis are slightly shorter than those of MgB2.
Structure and synthesis of MgB2
MgB2 is a simple binary compound, belonging to hexagonal system, with A1B2 type simple hexagonal structure and p6/mmm space group. This structure contains a graphite like B layer. Between the two B atomic layers, there is a hexagonal closely packed mg atomic layer, and the Mg atom is at the center of the hexagonal formed by B and F. The plane spacing of boron atoms in MgB2 crystal is obviously larger than that of atoms, which makes the thermal expansion coefficient of c-axis larger than that of a-axis.
Principle and basic properties of MgB2 superconductivity
Compared with the existing complex oxide superconductors with a critical temperature of 160K, the critical temperature of MgB is not high. Why can it cause such a sensation and response? One is that it is similar to complex oxide high temperature superconductors Mgb: is a simple binary compound Is a standard isotropic class I superconserved body.

