Silicon Boride Powder(SiB6)

.jpg)


.jpg)
.jpg)



Product Name: silicon hexaborate
English Name: boron silicide (b6si); Boron silicide; Silicon boride; Hexaboron silicide
CAS:12008-29-6 [1]
EINECS:234-535-8
Molecular formula: b6si
Molecular weight: 92.952
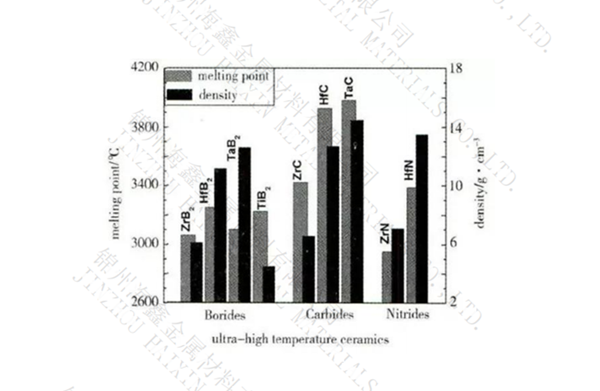
Relative density: 3.0g/cm3
Melting point: 2200 ℃
Application of silicon hexaborate:
1.B6si can effectively promote the sintering of boron carbide ceramics and
improve the mechanical properties of materials.
2. Because silicon hexaborate has heat resistance and high impact resistance,
it is relatively cheap, so it is often used in resin grinding wheels and as the
main abrasive.
Explanation of silicon hexaborate terms: chemical formula sib6. Molecular weight
92.95. Black crystal. The relative density is 2.47. The hardness is between diamond
and ruby. It can conduct electricity. Insoluble in water. When heated in chlorine
and steam, the surface can be oxidized. It can be oxidized directly in boiling nitric
acid. Unchanged in molten potassium hydroxide. It decomposes in hot concentrated
sulfuric acid. Explanation of preparation terms: the mixture of silicon and boron can
be directly heated, the excess silicon can be removed with hydrofluoric acid and nitric
acid, and then the silicon triboride (b3si) in the mixture can be decomposed with
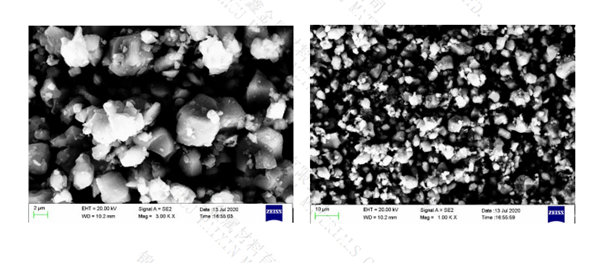
molten potassium hydroxide. The crystal structure of sib6 consists of interconnected
icosahedron (20 faceted polyhedron), icosahedron (26 faceted polyhedron) and isolated
silicon and boron atoms. Due to the size mismatch between silicon and boron atoms,
silicon can be used to replace boron in B12 icosahedron until the limit stoichiometric
ratio corresponding to sib2.89 is reached.
High purity silicon hexaborate process:
A low-cost and high-purity silicon hexaborate production process, boron trioxide and
potassium borohydride, in argon protection, after ball milling and mixing, are pressed
into blocks, loaded into a vacuum carbon tube furnace, evacuated, and maintained at
750 ℃ for 5 hours; Raise the temperature to 1250 ℃ and continue to maintain the
temperature until the pressure in the furnace is slightly positive, and the reduction
reaction is completely completed. Put the mixture of monomer boron and potassium
hydroxide in the graphite crucible into distilled water for heating, cleaning and drying
to obtain boron powder; The boron powder and silicon powder are milled and mixed,
and then loaded into the self creeping reaction kettle. The reaction kettle is evacuated,
and the vacuum degree is 1 Pa. The tungsten wire is heated by electricity, and the
zirconium powder is ignited. The high-temperature self creeping reaction is carried out.
The reaction is completed, the temperature is reduced, and the impurities are removed
to obtain high-purity silicon hexaborate With boron trioxide and potassium borohydride
as raw materials, the raw material cost is relatively low, and the whole process is reasonable
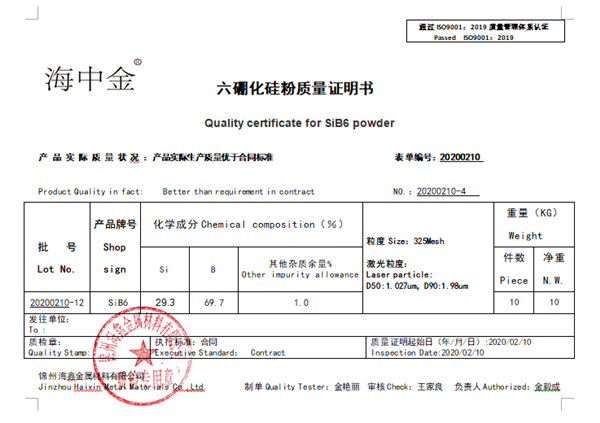
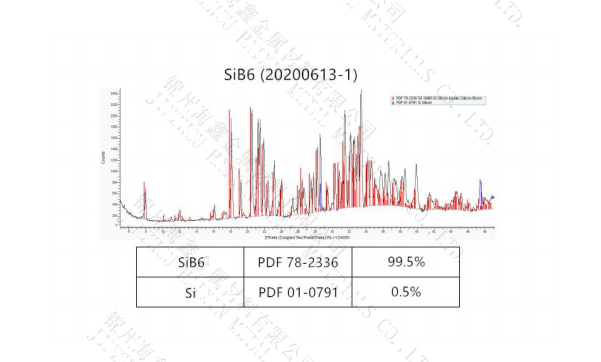
and controllable. The purity of silicon hexaborate produced is 99.5%, which is suitable for
industrial production.