Magnesium Silicide(Mg2Si)

.jpg)

.jpg)

.jpg)


.jpg)


Chinese Name: magnesium silicide
English Name: magnesium silicide
Chemical formula: Mg ₂ Si
Molecular weight: 76.71
CAS:22831-39-6
Melting point: 1102 ℃
Stability: it can be completely decomposed into simple substances when heated to 1200 ℃ in vacuum or under hydrogen flow. It is stable to alkali chemicalbook liquid, decomposes in acid and produces silane and hydrogen.
Density: 1.94g/cm ³
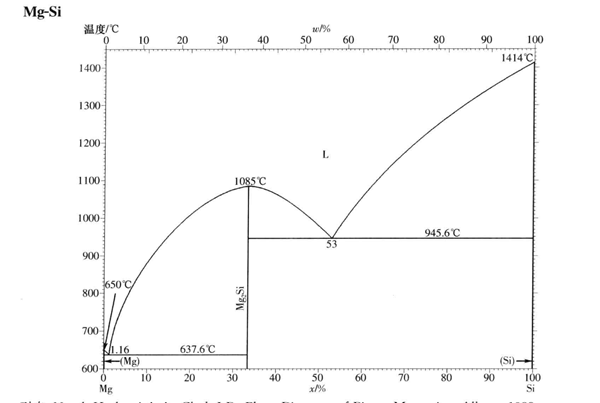
Mg2Si crystal has anti fluorite structure and belongs to face centered cubic lattice. The space group is fm3m, the group number is 225, and the lattice constant is a = 0.6391nm. In the crystal structure, each Si atom is located at (0,0,0) position, and the coordination number is 8, forming a face centered cubic structure with side length a. Mg atoms are located at (0.25,0.25,0.25) positions, and each mg atom is located at the center of the tetrahedron composed of Si atoms, forming a simple cubic structure with a side length of a / 2.
Preparation method:
Magnesium oxide (MgO) and silicon (SI) are formed when silicon dioxide (SiO2) and magnesium are heated together in a ratio of 1:2,
2 Mg + SiO2 → 2 MgO + Si
When magnesium is excessive, the remaining magnesium will continue to react with the generated silicon to form magnesium silicide, so the reaction of silicon dioxide to magnesium silicide can be expressed as follows:
4 Mg + SiO2 → 2 MgO + Mg2Si
When the mass ratio of magnesium to silicon dioxide is 1:4, silicon dioxide is completely converted into magnesium silicide. This reaction is very violent and will release a lot of heat. Magnesium silicide can also be obtained by reacting magnesium hydride and silicon at more than 250 degrees Celsius to generate hydrogen.
2 MgH2 + Si → Mg2Si + 2 H2
Crystal structure:
Mg2Si is a face centered cubic crystal with an anti fluorite structure, in which silicon atoms with a valence of - 4 are located at the vertex and face center of the cube, and Mg2 + ions are located at the eight tetrahedral vertices in the unit cell. There are still four gap defects in the unit cell.
Application:
1. Magnesium silicide (Mg2Si) is a stable compound of Mg Si binary system. It has the characteristics of high melting point, high hardness and high elastic modulus. It is a narrow band gap n-type semiconductor material. It has important application prospects in optoelectronic devices, electronic devices, energy devices, lasers, semiconductor manufacturing, thermostatic control communication, negative electrode materials for lithium-ion batteries, photovoltaic and other fields.
The semiconductor material magnesium silicide (Mg2Si) is a narrow band gap indirect semiconductor material. At present, the microelectronics industry is mainly based on Si materials. The process of growing Mg2Si thin films on Si substrates can be well compatible with the Si process, so the Mg2Si / Si Heterojunction structure has great research value. In this paper, magnetron sputtering is used to prepare environment-friendly Mg2Si thin films on Si substrates and insulating substrates respectively, and the effect of sputtering mg film thickness on the quality of Mg2Si thin films is studied. On this basis, the preparation process of Mg2Si based heterojunction LED devices is studied, and the electrical and optical properties of Mg2Si thin films are studied. First, Mg films were deposited on Si substrates, Si films and Mg films on insulating glass substrates by magnetron sputtering at room temperature, and then Mg2Si films were prepared by heat treatment in a low vacuum (10-1pa-10-2pa) atmosphere. XRD and SEM results show that Mg2Si thin films with single phase were prepared by annealing at 400 ℃ for 4h, and the prepared Mg2Si thin films have dense, uniform and continuous grains, smooth surface and good crystallinity. Secondly, the influence of Mg film thickness on the growth of Mg2Si semiconductor thin films and the relationship between Mg film thickness and the thickness of Mg2Si thin films formed after annealing were studied. The results show that the thickness of Mg film is in the range of 2.52 μ m、2.72 μ The thickness of Mg2Si thin film increases with the increase of Mg thickness, which is about 0.9-1.1 times of Mg thickness. This study will play an important role in guiding the design of devices based on Mg2Si thin films. After Z, the preparation of Mg2Si based heterojunction light emitting devices was studied. Mg2Si / Si and Si / Mg2Si / Si Heterojunction LED devices were prepared on Si substrates. The electrical and optical properties of Mg2Si / Si and Si / Mg2Si / Si heterojunctions were studied by using four probe test system, semiconductor characteristic analyzer, steady-state / transient fluorescence spectrometer and other equipment. The results show that the resistivity and block resistance of Mg2Si thin films decrease with the increase of Mg2Si thickness; Mg2Si / Si and Si / Mg2Si / Si heterostructures show good unidirectional conduction characteristics, and the conduction voltage of Si / Mg2Si / Si double heterostructures is relatively large, about 3V; The photoluminescence intensity of Mg2Si / n-Si heterojunction device is high at 1346 nm. The photoluminescence intensity of Mg2Si thin films prepared on insulating substrates is high at 1346 nm; Comparing the photoluminescence of Mg2Si thin films prepared on different substrates, the Mg2Si thin films prepared on high-purity quartz substrates have better luminescence performance and have infrared monochromatic luminescence characteristics.
2. Magnesium silicide is usually used to produce aluminum alloys. This alloy contains about 1.5% magnesium silicide and has aging hardening characteristics. It can form Guinier Preston region and very uniform precipitation, which can improve the strength of the alloy. When magnesium silicide is put into hydrochloric acid (HCl (AQ)), silane gas will be generated. If there is oxygen, silane will burn immediately.
Packaging and storage:
This product is packed in vacuum plastic bags, sealed and stored in a dry and cool environment. It should not be exposed to the air to prevent oxidation and agglomeration due to moisture, which will affect the dispersion performance and use effect; The number of packages can be provided according to the customer's requirements.