Lanthanum hexaborate (LaB6)

1、 Physical and chemical properties of LaB6
The existence range of lanthanum hexaborate: containing B 85.8-88 (wt)%, it is purple when containing B 85.8%, and blue when containing B 88%; Density is 4.7g/cm3, room temperature resistance is 15-27 μ Ω, Vickers hardness is 27.7 GPa, work function is 2.66 eV, emission constant is 29A/cm2 · K2.
Lanthanum hexaborate is opaque and appears light reddish purple when dry, and deep red when moist.
Lanthanum hexaborate has a cubic crystal structure, as shown in Figure 1:
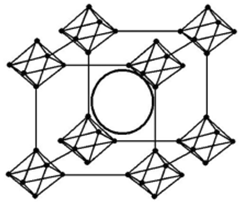
Figure 1 Crystal structure of LaB6
From the figure, it can be seen that the structural characteristics of the cubic crystal of lanthanum hexaborate are:
1) Boron atoms form a three-dimensional cubic framework structure, containing larger lanthanum atoms.
2) The boron framework is an octahedron, and at each vertex of a cube, there is an octahedron formed by a boron atomic framework, which is connected to each other by its vertices.
3) Each boron atom is adjacent to five boron atoms, four within its octahedron and one in the direction of one of the main axes of the cube, thus giving a homopolar lattice structure with a coordination number of 5.
4) Each boron atom has three valence electrons assigned to five bonds.
5) The coordination number of metal atoms trapped in a boron lattice is 24.
The crystal structure of borides determines their unique properties:
1) Due to the strong bonding force between boron atoms (lattice constant 4.145?), it is a refractory compound with a melting point of 2210 ℃.
2) The chemical properties are very stable and do not react with water, oxygen, or even hydrochloric acid; At room temperature, it only reacts with nitric acid and aqua regia; Oxygen only undergoes oxidation at 600-700 ℃.
3) Within a certain temperature range, the coefficient of expansion approaches zero.
4) Good stability in the air, and surface contamination during use can be restored by vacuum heat treatment.
5) Good resistance to ion bombardment and able to withstand high field strength.
6) Due to the absence of valence bonds between metal atoms and boron atoms, the valence electrons of metal atoms are free. So borides have high conductivity, and the resistance of lanthanum hexaborate is roughly the same as that of metallic lead. The temperature coefficient of its resistivity is positive.
7) If hexaborides are allowed to come into contact with refractory metals at high temperatures, boron will diffuse into the metal's lattice and form interstitial boron alloys with the metal. At the same time, the boron framework will collapse, allowing the metal atoms to evaporate.
8) When borides are heated to a certain temperature, the metal atoms on the surface of the crystal evaporate, but are immediately replenished by the metal atoms diffusing from inside the lattice, while the boron framework remains unchanged, minimizing the loss of surface active substances.
Due to the above advantages, LaB6 has been made into electronic components in modern technology and widely used in civil and defense industries: 1) Electronic emission cathodes. Due to the low electron escape work, cathode materials with the highest emission current at medium temperatures can be obtained, especially high-quality single crystals, which are ideal materials for high-power electron emission cathodes. 2) High brightness point light source. The core components used for preparing electron microscopes, such as optical filters, soft X-ray diffraction monochromators, and other electron beam light sources. 3) High stability and long lifespan system components. Its excellent comprehensive performance enables its application in various electron beam systems, such as electron beam engraving, electron beam heat sources, electron beam welding guns, and accelerators, for the production of high-performance components in engineering fields.
2、 Preparation of LaB6
(1) Preparation of LaB6 powder
1) Pure element synthesis method
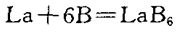
This method is the initial research method, suitable for phase diagram research, but not suitable for practical production applications.
2) Synthesis of compounds containing La and B
This method is an industrial method, and there are different reaction formulas depending on the reactants:
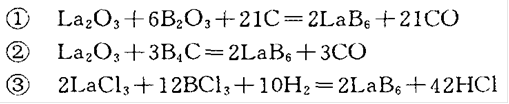
3) Reducing La compounds with pure B
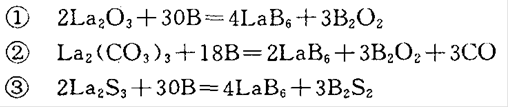
(2) Preparation of LaB6 polycrystalline materials
LaB6 polycrystals are generally prepared by sintering and hot pressing methods. In situations where the sample has voids, sintering can only be used for preparation. Sintering using LaB6, ZrB2, or ZrC crucibles. To prevent the infiltration of B, it is not advisable to use B crucible. Usually sintered in a hydrogen atmosphere. The hot pressing pressure is 400 atm, the temperature is 2000 ℃, and the holding time is 1-2 hours. The size of the billet is generally φ 100mm x 30mm.
(3) Preparation of LaB6 Single Crystal
At present, the preparation methods of single crystals can be summarized as zone melting method, solvent method, and gas-phase method.
1) Zone melting method
Zone melting method is the most commonly used method for preparing rare earth boride single crystals. When using LaB6 as an electrode radiation material, it is necessary to prepare single crystals with high purity. Although no exact relationship has been found between the impurities in LaB6 and its service life as an emitting electrode, the higher the purity of LaB6, the longer its service life. Therefore, preparing high-purity materials is very meaningful.
In order to prepare high-purity LaB6, a suspension zone melting method without a crucible is generally adopted, protected by inert gas, as shown in Figure 2:
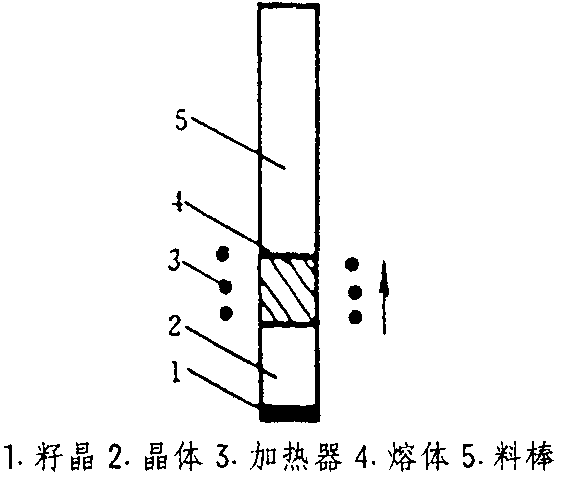
Figure 2 Schematic diagram of zone melting method
The zone melting methods for preparing single crystals include radio frequency heating, electron beam heating, arc heating, and laser beam heating.
2) Solvent method
The solvent method is also the basic method for preparing single crystal LaB6, which includes two methods: aluminum solvent method and rare earth solvent method. The two are similar, except that the latter uses rare earth elements instead of aluminum, as shown in the diagram below:
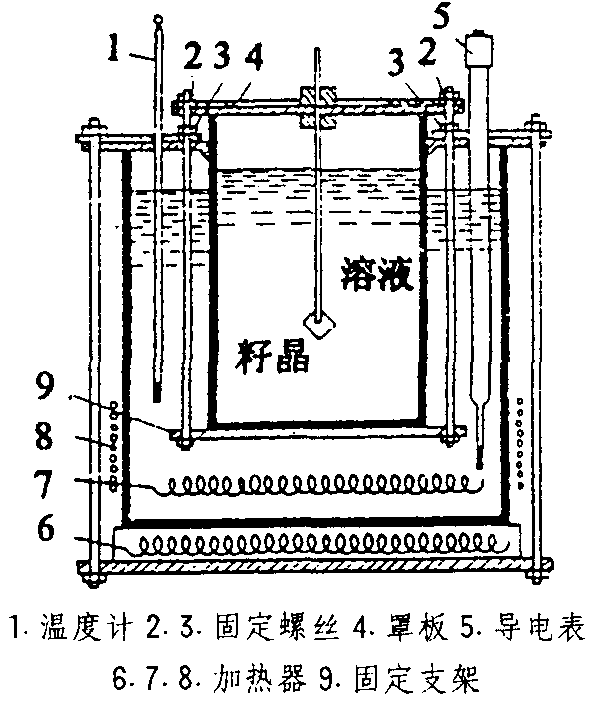
Figure 3 Schematic diagram of aluminum solvent method
3) Gas phase precipitation (CVD) method
Gas phase precipitation method is the process of using gaseous substances to undergo chemical reactions on the surface of a solid material, generating solid deposits. The schematic diagram of its principle is as follows:
Figure 4 Schematic diagram of CVD method principle
The chemical reaction formulas applicable to the production of LaB6 by CVD method include:
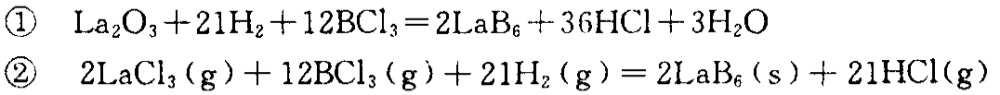
3、 Emission properties of single crystal LaB6 cathode
LaB6 cathode is a special crystal structure that, due to its good conductivity and low escape work of metals, can obtain a DC emission current of 0-100A/cm2 when operating at 1400-1680 ℃, far superior to oxide and pure metal cathodes; At the same time, it also has good thermal and chemical stability.
Since the discovery that LaB6 has a low work function and low vapor pressure, making it suitable as a material for hot electron emission, people have been studying the advantages of LaB6 cathodes. Previously, sintered LaB6 was used, but it had issues with brightness and stability due to its uneven structure and high impurities. These problems were solved after the development of single crystal LaB6, as it has a uniform structure and high purity. Therefore, single crystal LaB6 has replaced hairpin tungsten cathodes for electron beam devices such as electron microscopes and electron exposure systems.
Research has shown that the (100) crystal plane has the lowest work function, can provide the highest current density, and is best used.
(1) The influence of cathode tip size
In theory, the brightness of the electron beam emitted from a hot electron gun is determined by the cathode temperature, work function, and acceleration voltage. However, the actual brightness is reduced due to emission current limitations and space charge effects. The space charge effect depends on the field strength distribution in the electron gun space, which depends on the geometric shape and size of the cathode surface, including the size of the cathode tip.
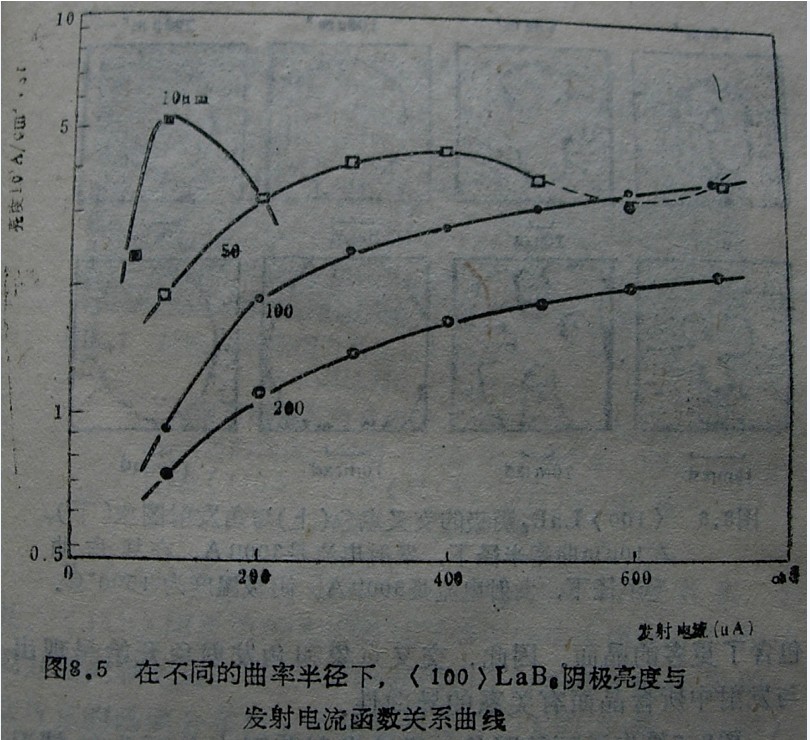
The experiment indicates that when the tip curvature is relatively small, the high brightness desired by the low work function LaB6 can be obtained. However, during long-term working hours, the small spherical surface at the tip of the cathode with a small curvature radius only disappears after a shorter working time. one hundred μ After more than 1500 hours, the large curvature radius of m can still maintain a (100) plane perpendicular to the optical axis, providing a relatively stable emission.
(2) The influence of tip axis orientation
The anisotropy of the work function depends on the lattice structure of LaB6 crystal, therefore, the emission characteristics also depend on the crystal orientation along the cathode tip axis. It is important to select the appropriate orientation as the axis of the cathode tip in order to obtain electron emission suitable for electron beam devices.
The experiment shows that LaB6 has the lowest energy dissipation in the<100>crystal direction, so it has the highest brightness. Therefore, it is required that the axis of the cathode rod should be consistent with this crystal direction, with an error of ± 2 °.
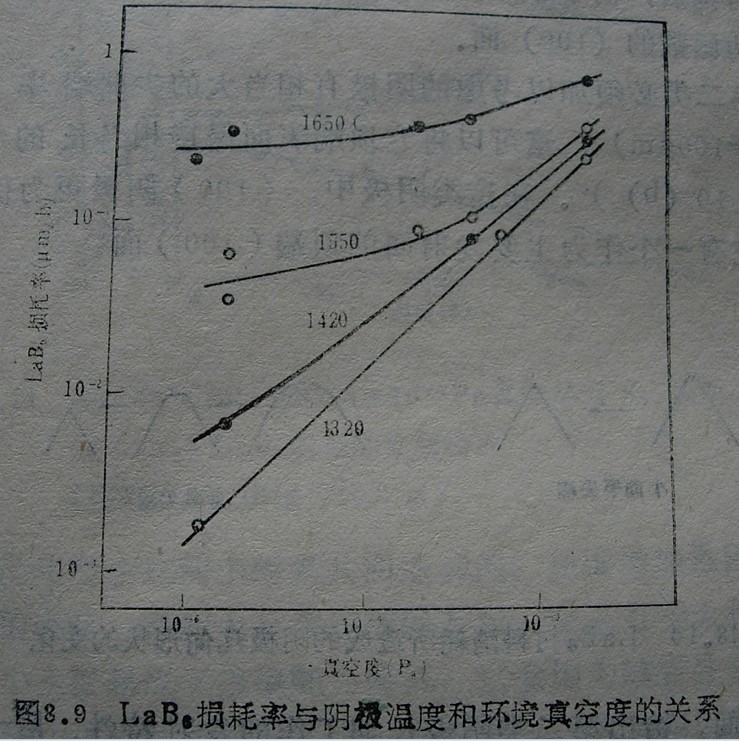
(3) Lifetime characteristics
The lifespan characteristics of LaB6 cathode, that is, the emission performance of long-term operation, are mainly determined by the consumption of LaB6 material. The consumption of LaB6 is caused by the thermal evaporation of the material, which increases with the increase of temperature and the deterioration of vacuum degree.