Study on photocatalytic hydrogen production of nickel borate modified titanium dioxide
Hydrogen is considered as one of the most attractive and developing energy sources, which can effectively alleviate energy shortage, environmental pollution and other issues related to climate change. Since Honda and fujishim first discovered that TiO2 can be decomposed into water under UV irradiation in 1972, photocatalytic hydrogen production technology has gradually become a research hotspot. Among a large number of hydrogen production technologies, semiconductor based photocatalysis technology has more potential, economic, renewable and safe. TiO2 is widely used in the field of photocatalytic hydrogen production because of its biological and chemical inertness, strong oxidation-reduction ability, environmental friendliness and low cost. The actual photocatalytic hydrogen production efficiency of pure TiO2 is not high due to the slow electron transport, the fast electron hole recombination speed and the lack of effective hydrogen production active sites on the surface. Improving the transfer rate of photogenerated charge on the TiO2 surface, inhibiting the rapid recombination of photogenerated electrons and holes on the TiO2 surface and improving the effective utilization of electrons are the keys to improve its photocatalytic efficiency.
The introduction of cocatalysts on the surface of semiconductors is considered to be an effective means to improve the photocatalytic efficiency of materials. The supported cocatalyst can promote the migration and separation of carriers, and provide active sites for the surface photocatalytic reaction. Generally, supported noble metal cocatalysts, such as Pt, Ru and Au, can effectively promote the separation of photogenerated electrons and holes, reduce the overpotential of hydrogen production, and achieve the purpose of promoting photocatalytic hydrogen production. However, the high cost and low natural abundance of noble metal cocatalysts seriously limit their large-scale application. Therefore, it is very important to find a low-cost and rich alternative cocatalyst. So far, various non noble metal carbides, sulfides and phosphides have been proved to be efficient cocatalysts to promote photocatalytic hydrogen production. However, so far, eco-friendly transition metal borides (TMB) have not been widely used as Co catalysts for photocatalytic hydrogen production. At present, it has been reported that transition metal borides (mob, FeB2, NiFeB, ni3b) have excellent electrocatalytic hydrogen production capacity, mainly because TMB can achieve effective electron transfer, and the active boron in borides can protect the active metal sites from oxidation. For example, Cao et al. Introduced ni2b nanoparticles into g-c3n4. Ni2b acts as an insulator between carbon nitride layers to increase the specific surface area, reduce the thickness of the layer, and also acts as a conductive material to further improve the conductivity of the material. At the same time, the synergy between carbon nitride and ni2b helps to reduce the adsorption energy of intermediates and the over potential required for Electrocatalysis, and finally improves the electrocatalytic hydrogen production activity of carbon nitride.
In recent years, TMBs have been gradually studied as a cocatalyst for photocatalytic hydrogen production. For example, Li et al. Constructed a ternary photocatalyst system composed of graphene, CdS nanoparticles and transition metal borides, in which amorphous transition metal borides (NIB or NiCoB) were used as cocatalysts, and their maximum visible light photocatalytic hydrogen production activity reached 144.8 mmol h − 1g − 1, 36 times that of pure CDs. However, due to the high toxicity and low stability of CDs, these TMB modified CDs composite systems can not be widely used in practical photocatalytic hydrogen production. Therefore, in this paper, amorphous nickel borate is introduced as an environmental friendly cocatalyst to modify environment-friendly titanium dioxide, and the photocatalytic hydrogen production performance of the material is studied. At the same time, combined with the photoelectric performance changes of the material before and after the introduction of cocatalyst, the effect of the introduction of cocatalyst on the activity improvement is discussed.
2. experiment
2.1. Experimental reagent
Nickel acetate tetrahydrate (Ni (CH3COO) 2 ¤ 4H2O), ethanol (C2H5OH), methanol (CH3OH), sodium sulfate (Na2SO4) and potassium chloride (KCl) are all purchased from the chemical reagent of Sinopharm group. Potassium ferricyanide (k3[fe (CN) 6]) and potassium ferrocyanide (k4[fe (CN) 6]) are purchased from Aladdin reagent. Nafion solution was purchased from Beijing honghaitian Technology Co., Ltd. Titanium dioxide (P25) was purchased from Degussa. Sodium borohydride (NaBH4) was purchased from Shanghai Lingfeng reagent. All the above reagents are analytically pure and can be used directly without any other treatment.
2.2. Preparation of catalyst
First, weigh 0.0249 g (0.1 mm) of nickel acetate and dissolve it in 40 ml of deionized water, and continue stirring. Weigh 0.0113 g (0.3 mm) of sodium borohydride and add it to 10 ml of ice water. Add the NaBH4 solution slowly to the nickel acetate solution. During the dropping process, the color of the solution gradually turns black, and bubbles constantly emerge. Keep the ice water bath during the reaction. After continuous stirring until no bubbles are generated, wash with deionized water and ethanol, centrifuge, and vacuum dry at 30 ℃ to obtain sample nib. For the preparation of nib/p25, 0.5g P25 shall be added to the prepared nickel acetate solution. Other operation steps are the same as above. The sample obtained by vacuum drying is recorded as NB-1. The preparation process of nib/p25 sample is shown in Figure 1. By changing the amount of nickel acetate and sodium borohydride added, the obtained samples are marked as NB-3, nb-5 and nb-7, and the specific parameters are shown in Table 1. Commercial P25 uses sodium borohydride for the same treatment. The treatment process is as follows: add 0.5 g of p25 into 40 ml of deionized water, continuously stir and maintain an ice water bath. Weigh 0.0113 g of sodium borohydride and dissolve it in 10 ml of ice water. Slowly add the prepared NaBH4 solution to the mixture of p25, wash with deionized water and ethanol, and centrifuge and dry to obtain the treated sample.
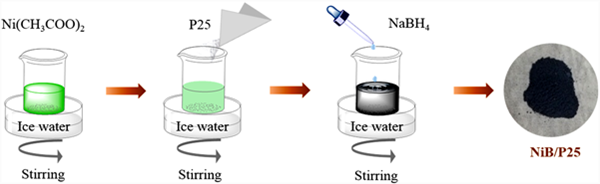
Figure 1. Schematic diagram of the preparation process of NiB/P25 samples
样品编号 | Ni(CH3COO)2∙4H2O (g) | NaBH4 (g) | P25 (g) |
P25 | 0 | 0.0113 | 0.5 |
NiB | 0.0249 | 0.0113 | 0 |
NB-1 | 0.0249 | 0.0113 | 0.5 |
NB-3 | 0.0747 | 0.0340 | 0.5 |
NB-5 | 0.1245 | 0.0567 | 0.5 |
NB-7 | 0.1742 | 0.0794 | 0.5 |
Table 1. The raw material components of NiB/P25
2.3. Characterization of catalyst
The samples were powder X-ray diffraction (XRD) using Cu K α The Bruker AXS D8 discover powder X-ray diffractometer (Brooke, Germany) of the radiation source was used for testing. The UV Vis diffuse reflectance spectra of the samples were tested with Hitachi uh4150 solid-state UV diffuse reflectometer (Hitachi, Japan). Geminisem 300 field emission scanning electron microscope (Carl Zeiss, Germany) was used to analyze the morphology of the samples, and the energy dispersive spectrometer (EDS) was equipped to determine the composition of the samples. Use Hitachi f4600 fluorescence spectrophotometer (Hitachi, Japan) to test the fluorescence emission spectrum (PL) of the sample.
2.4. Photocatalytic hydrogen production activity test of samples
The photocatalytic hydrogen production activity test was carried out in the illuminated glass reaction vessel connected to the closed glass gas circulation system (Zhongjiao Jinyuan, Beijing, cel-sph2n). Using a 300 W full spectrum xenon lamp as the light source (1 cm away from the photocatalytic reactor), add the photocatalyst (0.01 g) to the reactor containing 100 ml of methanol aqueous solution (20 vol%). Before light irradiation, vacuum the reactant solution with a vacuum pump to remove the residual air in the system and the dissolved oxygen in the reaction solution. During the photocatalytic reaction, the temperature of the reaction solution is kept at 10 ℃ by flowing cooling water. The amount of hydrogen produced is determined by an on-line gas chromatograph (Zhongjiao Jinyuan, Beijing, gc7920). The chromatograph is equipped with a thermal conductivity detector (TCD) and a 5 Å molecular sieve column, using argon as the carrier gas.
2.5. Photoelectric performance test
The photoelectrochemical test of the sample was carried out on the Chi 760 electrochemical workstation (Shanghai Chenhua). In the traditional three electrode system, Pt wire was used as the counter electrode and calomel electrode as the reference electrode. Apply 80 µ l of 10 mg/ml sample suspension (add 0.01 ml of Nafion membrane solution to each ml of suspension) to 1 × 1 cm − 2 indium tin oxide (ITO) conductive glass substrate is used as the working electrode and dried naturally. Using 0.2 mol/l sodium sulfate solution as electrolyte and 50 W full spectrum xenon lamp as light source, the photocurrent response curve was obtained by irradiating at fixed intervals. The linear sweep voltammetric curve (LSV) was measured with ag/agcl electrode as the reference electrode and ITO conductive glass sheet coated with the sample as the working electrode in the scanning range of 0 ~ − 1.0 V and the scanning speed of 50 MV • s − 1. Electrochemical impedance spectroscopy (EIS) is to apply 8 µ l sample suspension to the glassy carbon electrode as the working electrode, with 25 mmol/l mixed potassium ferricyanide / potassium ferrocyanide solution as the electrolyte, in which 0.1 mol/l potassium chloride is added, and the test frequency range is 1~10 kHz.
3. results and discussion
3.1. Characterization of catalyst
Fig. 2 (a) shows the powder X-ray diffraction spectrum of NIB sample. There is no sharp crystallization peak in the figure, indicating that the prepared catalyst is an amorphous substance with low crystallinity. Of which, 2 θ = forty-five ˚ The wide peak at belongs to amorphous nickel borate, 2 θ = twenty-two ˚ The nearby small bulges are attributed to amorphous boron oxide, which is reported to be formed during the reduction reaction in aqueous solution. Heat treat the prepared amorphous NiB sample in N2 atmosphere at 250 ℃ for 3 hours. Figure 2 (b) shows the powder X-ray diffraction spectrum of the heat treated sample. Some sharp crystallization peaks appear in the figure, indicating that the heat treatment improves the crystallinity of NIB sample, and these diffraction peaks can be consistent with the standard card of ni3b, indicating the successful preparation of nickel borate sample. Fig. 3 is the SEM-EDX image of the prepared NIB sample. The SEM image shows that the nib sample is fluffy Tremella like. The composition elements and distribution of the sample are analyzed in combination with X-ray energy spectrum analysis (EDX). Ni and B elements are evenly distributed on the material, which is consistent with the XRD results.
Figure 4 shows the powder X-ray diffraction patterns of p25 and nib/p25 samples. All diffraction peaks in the figure can correspond to the standard cards of two different crystal forms of titanium dioxide in P25, namely tetragonal anatase (ICSD 00-004-0477) and rutile (ICSD 01-076-0318), indicating that the preparation process has not affected the crystal structure of p25. There is no excess diffraction peak except TiO2 in the spectrum, and no significant difference is observed between the spectra of each sample, which may be due to the low content and low crystallinity of the introduced nib. The composition of the sample was analyzed by scanning electron microscope and energy dispersive X-ray (EDX). Figure 5 shows the scanning electron microscope image and element distribution diagram of NB-3 sample. The results show that the sample contains Ti, O, Ni and B elements, and the distribution density of Ti and O elements is significantly higher than that of Ni and B elements. It is confirmed that the sample is a nib modified P25 composite
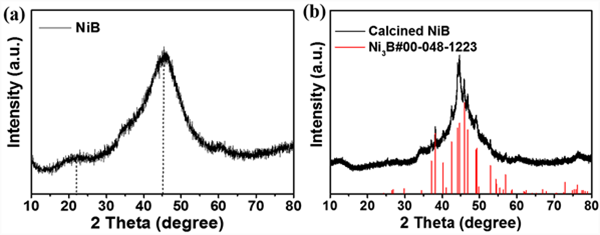
Figure 2. XRD pattern of NiB before (a) and after calcination (b)
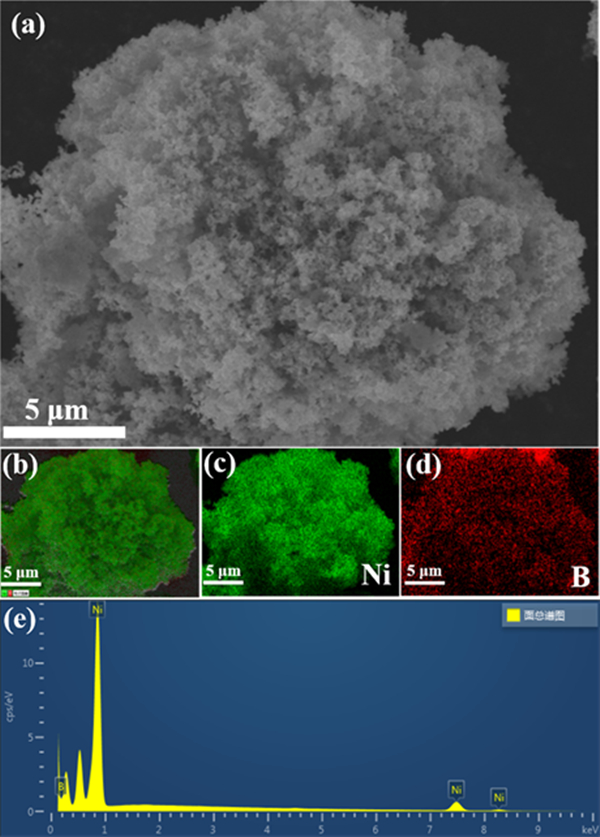
Figure 3. The FESEM image (a), selected area EDX elemental mappings of Ni, B (b-e) of NB-3
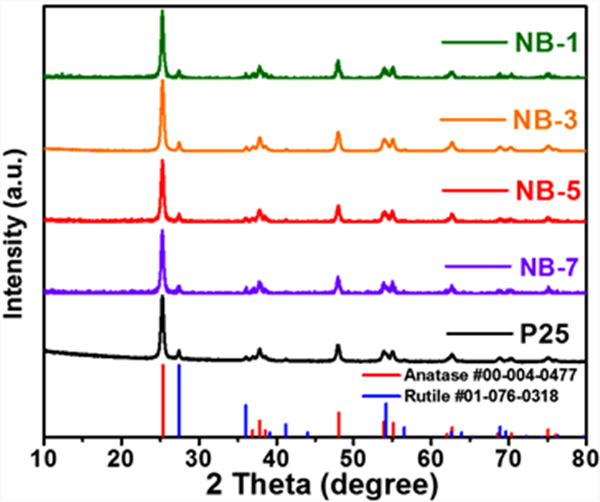
Figure 4. XRD pattern of P25 and NiB/P25
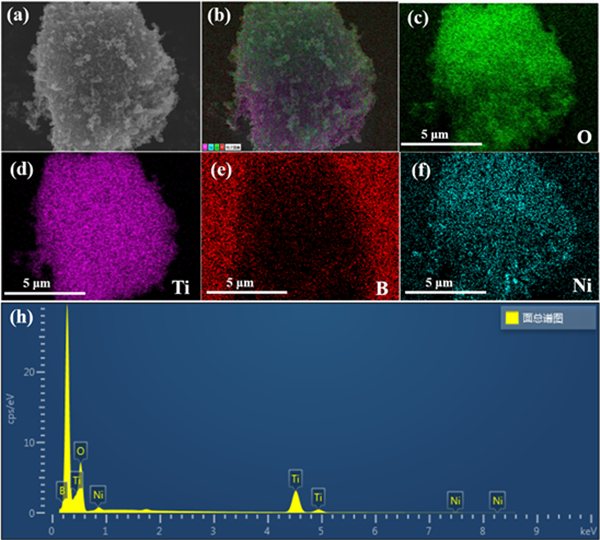
Figure 5. The STEM image (a) and selected area EDX elemental mappings of Ti, O, Ni, B (b-f) of NB-3
Fig. 6 (a) shows the UV Vis diffuse reflectance spectra of nib, P25 and nib/p25 samples. NIB shows a high light absorption. Except nib, other samples show similar absorption edges at about 400 nm, and with the increase of the amount of NIB introduced, the color of the samples also gradually deepens (Fig. 6 (b)), the absorption intensity of nib/p25 samples increases, and the absorption edge of the material gradually redshifts. This means that the introduction of NIB improves the light response range of the sample, increases the light absorption of the material, and enables the sample to absorb more photons, thus producing more photogenerated electron hole pairs.
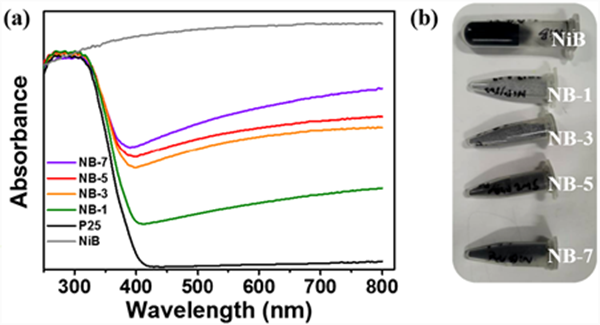
Figure 6. UV-Vis diffuse reflectanse spectra (DRS) (a) and image of samples (b)
3.2. Photocatalysis performance of catalyst
Using 20 vol% methanol as sacrificial agent, the photocatalytic hydrogen production performance of the sample was measured under full spectrum light irradiation (Fig. 7). As shown in Fig. 7 (a) and Fig. 7 (b), the control experiment results show that the photocatalytic hydrogen production of pure P25 is less, and the total hydrogen production rate in 4 hours is only 24 µ mol g − 1 h − 1. The nib modified samples showed significantly improved photocatalytic hydrogen production activity. With the increase of NIB modified amount, the photocatalytic hydrogen production activity of the samples increased first and then decreased. Among them, NB-3 has the highest hydrogen production activity. The total hydrogen production rate in 4 hours is 2651.6 µ mol g − 1 h − 1, about 110 times that of pure P25 under the same conditions. Under the same test conditions, the photocatalytic hydrogen production performance of pure nib and P25 samples treated with NaBH4 was tested. As shown in Fig. 7 (c) and Fig. 7 (d), it was found that pure NIB samples did not have the ability of photocatalytic hydrogen production, indicating that NIB only played a role as a cocatalyst after it was introduced. P25 after NaBH4 treatment showed very low photocatalytic hydrogen production activity, and the hydrogen production rate within 4 hours was only 17 µ mol g − 1 h − 1, indicating that the change of p25 carrier after sodium borohydride treatment did not promote the photocatalytic hydrogen production activity, and the increase of photocatalytic activity of the sample was due to the introduction of nib. It is worth noting that the photocatalytic hydrogen production activity of NB-3 sample decreased significantly after heat treatment in N2 atmosphere at 250 ℃ for 3 hours, indicating that amorphous nib is more conducive to promoting the photocatalytic hydrogen production performance of p25.
The stability of nib/p25 photocatalyst was tested by 24-hour continuous photocatalytic hydrogen production experiment. NB-3 was used as the experimental object, 20 vol% methanol was used as the sacrificial agent, and the full spectrum light was irradiated for 24 hours. No sacrificial agent was added during the reaction. The test results are shown in Fig. 7 (E). The photocatalytic hydrogen production of NB-3 sample increases steadily with the extension of illumination time, which indicates that the material has good photo corrosion resistance. In Figure 7 (f), the hydrogen production rate of the sample increases rapidly within 0~5 hours, and then decreases slightly and gradually becomes stable. This may be due to the rapid combination of the photocatalyst and the sacrificial agent molecules in the solution in the first few hours, and the rapid generation and removal of hydrogen on the material surface. Thereafter, the combination and departure rates of the two gradually reach equilibrium, and the hydrogen production rate becomes stable, The slight decrease of hydrogen production rate may be due to the continuous consumption of sacrificial agent molecules in the solution. The experimental results of 24-hour continuous photocatalytic hydrogen production show that nib/p25 material has good stability in the process of photocatalytic hydrogen production.
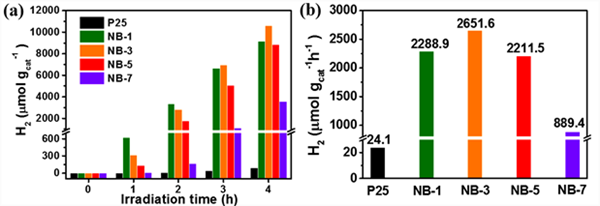
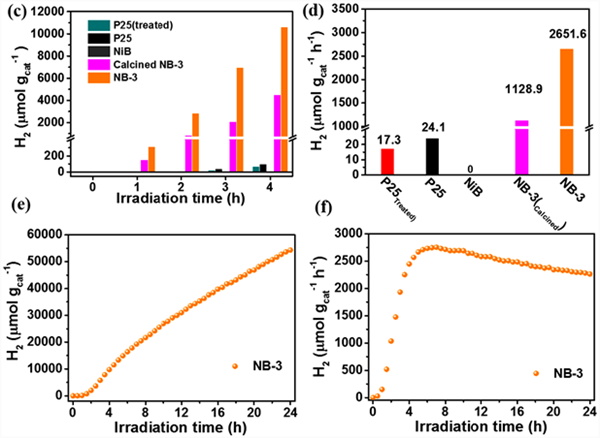
Figure 7. The plots of H2 production versus time and total H2 evolution rate of samples (a - f)
3.3. Mechanism of photocatalytic hydrogen production
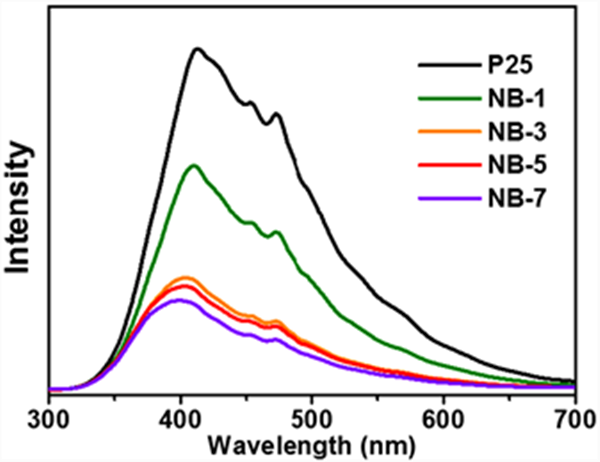
The separation and migration of photogenerated charges were analyzed by the fluorescence (PL) spectra of the samples. Fig. 8 shows the fluorescence spectra of p25 and nib/p25 under 250 nm excitation light. It can be found that the samples before and after modification have the same fluorescence emission peak type, which is mainly from the fluorescence generated by electron hole recombination after P25 excitation. However, the fluorescence intensity of the sample after NIB introduction is significantly reduced, which indicates that after NIB introduction, electrons can be transferred from TiO2 to nib, inhibiting electron hole recombination, thus reducing the fluorescence intensity, and this part of the electrons transferred to nib can be used for subsequent photocatalytic hydrogen production reaction, which is also the main reason for the improvement of its activity after NIB introduction. However, it should be noted that nb-7 has the lowest fluorescence intensity, which is not consistent with the active order of photocatalytic hydrogen production, indicating that the carrier separation efficiency is not the only factor affecting the photocatalytic activity.
The effect of nib on the improvement of charge separation and migration was further studied by measuring the transient photocurrent response and electrochemical impedance spectroscopy. Figure 9 (a) shows the transient photocurrent response curve of p25 and NB-3 samples at equal time intervals. Both P25 and NB-3 produce significant photocurrent signals under illumination. Compared with pure P25, the photocurrent density of the modified material increased significantly. This shows that the introduction of NIB cocatalyst can effectively promote the separation of carriers and enhance the concentration of electrons, which is consistent with the results of fluorescence spectra. Figure 9 (b) shows the electrochemical impedance spectra of p25 and NB-3. Compared with P25, the charge transfer resistance of NB-3 material is significantly smaller, which indicates that the introduction of high conductivity nickel borate reduces the resistance of the material and enhances the rate of charge transfer. It also indicates that the introduction of nickel borate can improve the rate of photocatalytic hydrogen production by promoting charge transfer. The linear sweep voltammetry (LSV) curve of the sample was tested to analyze the role of NIB cocatalyst in the photocatalytic reaction. Fig. 9 (c) shows the polarization curve of p25 and NB-3 samples during negative scanning from 0 to − 1.0 V (relative to ag/agcl). The results show that both samples have obvious cathode current, indicating that hydrogen evolution reaction (her) occurs. The cathode current density of the modified NB-3 is significantly increased, which means that the nib cocatalyst can greatly increase the electron concentration. Meanwhile, NB-3 shows a smaller initial potential than P25, which means that nib can reduce the overpotential of hydrogen production and effectively promote the proton reduction reaction.
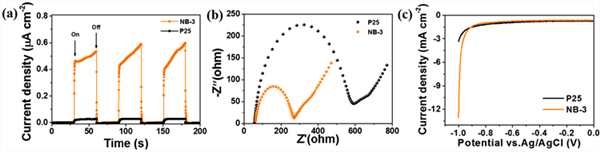
Figure 9. Photocurrent responses (a), electrochemical impedance spectroscopy (EIS) (b), and linear sweep voltammograms (LSV) (c) of sample
Based on the above experimental results, the possible photocatalytic hydrogen production mechanism of nib/p25 composite is proposed, as shown in Figure 10. Under illumination, P25 absorbs photons and excites to generate photogenerated electrons and holes. The electrons transition from valence band (VB) to conduction band (CB). At this time, most of the electrons in CB and the holes in VB rapidly compound and emit fluorescence. A small number of electrons and holes participate in reduction and oxidation reactions respectively, resulting in low photocatalytic hydrogen production activity of pure P25. However, after the introduction of nib, due to the close contact between P25 and nib, the photogenerated electrons transiting to the CB of p25 can be quickly transferred to the nib cocatalyst, which promotes the separation and transfer efficiency of electron hole pairs, inhibits the recombination of electrons and holes, reduces the fluorescence intensity, improves the effective utilization of photogenerated electrons, and finally significantly improves the photocatalytic hydrogen production activity of the material.
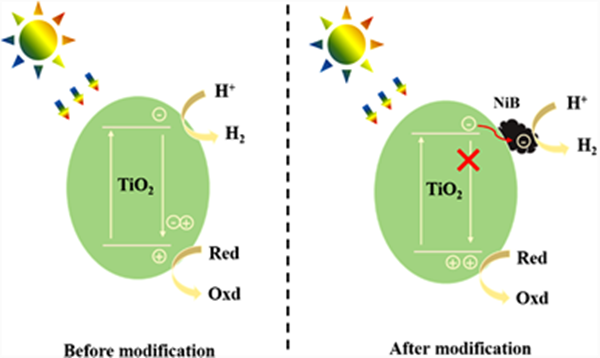
Figure 10. Schematic diagram of carrier migration in the photocatalytic hydrogen production process of P25 and NiB/P25 samples
4. conclusion
Using sodium borohydride as boron source, the nickel borate modified P25 material was synthesized by a simple method. The amount of NIB modification can be simply adjusted by adjusting the amount of boron source and nickel source. XRD results showed that NIB existed in amorphous state. The introduction of NIB significantly improved the photocatalytic hydrogen production activity of the material. The photocatalytic hydrogen production rate of the material with the best modification amount was 2651.6 µ mol g − 1 h − 1, about 110 times that of pure P25. The results of UV-Vis absorption spectra, fluorescence spectra, photocurrent response, electrochemical impedance and linear sweep voltammetry show that NIB modification improves the photocatalysis response of the material, promotes the separation of photogenerated electrons and holes, reduces the resistance of carrier migration, and reduces the overpotential of hydrogen production, thus greatly promoting the photocatalytic activity of the material. In addition, the 24-hour continuous photocatalytic hydrogen production test showed that the catalyst had good stability.

