Research progress of boride ultrahigh temperature ceramics
Modern aircraft, such as spaceships, artificial satellites, rockets, missiles, supersonic aircraft, are developing towards the direction of high thrust, high speed and long distance, which puts forward higher requirements for the high temperature resistance of materials. For example, the nose cone of rocket and the leading edge of supersonic aircraft wing need to work in a neutral or oxidizing environment of 2000-2400 ℃. This makes the development of ultra-high temperature materials more and more urgent. The traditional ultra-high temperature materials mainly include the following three types: refractory metal materials and c/c composites represented by niobium, tantalum, tungsten and molybdenum, and ultra-high temperature ceramic materials (UHTCs) represented by transition metal borides, carbides and nitrides. Refractory metal materials have good high-temperature mechanical properties and are easy to be processed and formed, but they are prone to oxidation at high temperatures. For example, niobium alloys will undergo catastrophic oxidation and spalling in air above 400 ℃. C/c composites have high strength, high modulus, good fracture toughness and wear resistance, but they are prone to oxidation in the air environment above 370 ℃.
The application of the above two ultra-high temperature materials in extreme aviation environment requires the development of corresponding high-temperature oxidation resistant coatings. The melting point of ultra-high temperature ceramic materials is very high, such as HfB2, which can reach 3250 ℃, with high hardness, good chemical stability, and excellent high-temperature oxidation resistance [3]. This series of advantages makes ultra-high temperature ceramic materials not only can be used independently as ultra-high temperature structural materials, It can also be used as the anti-oxidation coating of the first two high-temperature structural materials. Among the numerous ultra-high temperature ceramic materials, boride ultra-high temperature ceramic materials TiB2, HfB2 and ZrB2 are considered as the ultra-high temperature ceramic materials with the best oxidation resistance and become a research hotspot.
I Physical properties of boride ultrahigh temperature ceramics
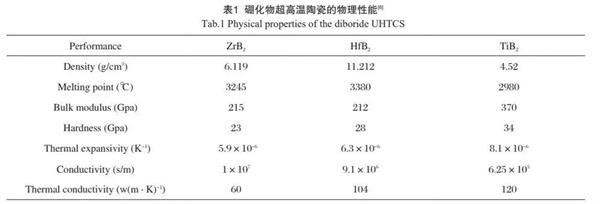
Boride ultra-high temperature ceramics have high melting point and good chemical stability. Compared with other ultra-high temperature ceramics, boride ceramics have high conductivity, high thermal conductivity and good corrosion resistance. In the high temperature oxidation environment, the oxidation reaction MB Ω +o Ω → Mo Ω +b Ω o Ω occurs, and the products are glass phase B2O3 and metal oxide Mo Ω. Some of their physical properties have been summarized in Table 1.
II Thermodynamic analysis of oxidation behavior of boride ultrahigh temperature ceramics
Taking TiB Ψ ultra-high temperature ceramics as an example, TiB Э may react with O Э in the following ways under the condition of high temperature oxidation. The standard Gibbs free energy of each reaction equation can be calculated by referring to the thermodynamic data table Δ Relationship between go and temperature T:

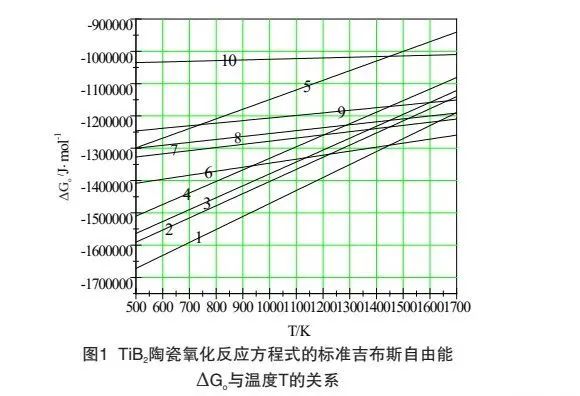
It can be seen from Figure 1 that under the oxidation environment with the reaction temperature between 500k-1700k, the standard Gibbs free energy of reaction (1) to reaction (10) is less than 0, and the above reactions are likely to reverse. The oxidation kinetic conditions of TiB Ψ materials in the same environment are similar. When the reaction temperature is between 500K and 1450k, the standard Gibbs free energy of reaction (1) is the smallest, and the oxidation trend according to reaction (1) is the largest. When the temperature exceeds 1450k, the standard Gibbs free energy of reaction (6) is the smallest, and the oxidation trend according to reaction (6) is the largest. Reaction (1) and reaction (6) are combined to obtain reaction (11):
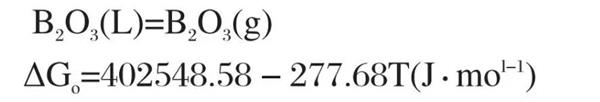
When the temperature t=1450 K, the standard Gibbs free energy of reaction (11) is less than 0, and the reaction (11) starts. However, in actual air oxidation, pb‹o ₃ is much lower than the standard atmospheric pressure. According to van der Hoff isothermal formula:
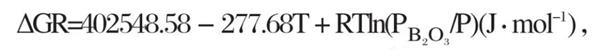
Rtln (pb‹o ₃ /p) <0, make Δ Gr=0, t=402548.58/ (277.68 - RLN (Pb Ψ o ₃ /p)), t<1450 K. therefore, in the atmosphere of air oxidation, when the temperature is lower than 1450 K, the reaction formula (11) begins to occur. From the above inference, we can know that the following two reactions mainly occur when TiB2 ceramics are oxidized in high temperature air:
When the temperature is low, the reaction formula is:
TiB₂(s) + 5/2O₂(g)=TiO₂(s) + B₂O₃(L);
When the temperature is high, the reaction formula is:
TiB₂(s) + 5/2O₂(g)=TiO₂(s) + B₂O₃(g)。
III High temperature protection mechanism of boride ultrahigh temperature ceramics
The oxidation behavior of boride UHT ceramics is different in different temperature ranges. We usually divide the oxidation temperature range into three temperature ranges, taking ZrB Ψ as an example, respectively (1) (2) (3)
(1) T<1000℃;
(2) T=1000-1800℃;
(3) T>1800℃。
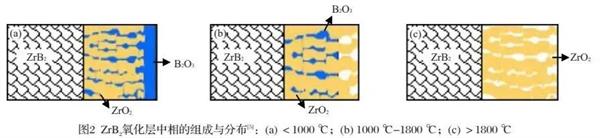
In the low temperature range of t<1000 ℃, the oxide layer is mainly composed of porous refractory metal oxide skeleton ZrO Ψ and glass phase b2o ₃. The glass phase B Ψ o ₃ is filled in the refractory metal oxide skeleton. The surface of the oxide layer is covered with a layer of glass phase B Ψ o ₃ with good fluidity. Oxygen is directly dissolved in the glass phase and diffused to the interface between the matrix and the oxide layer to produce oxidation reaction; In the medium temperature range of t=1000 ℃ -1800 ℃, the oxide layer is also mainly composed of porous refractory metal oxide skeleton ZrO Ψ and glass phase B Ψ o ₃, which is filled in the refractory metal oxide skeleton. However, at this time, the surface of the oxide layer is a bare refractory metal oxide skeleton, and oxygen first reaches the glass phase through the refractory metal oxide skeleton on the surface of the oxide layer, Then it is dissolved in the glass phase and diffused to the interface between the substrate and the oxide layer to produce oxidation reaction; In the high temperature range of t>1800 ℃, oxygen directly reaches the oxidation substrate at the interface between the substrate and the oxide layer through the connected holes in the refractory metal oxide skeleton.
The pores of the refractory metal oxide framework are filled with well flowable B Ψ O3 glass phase, both of which have low oxygen permeability, which hinders the diffusion of oxygen. In addition, the flowing B Ψ O3 glass phase can timely make up for the thermal shock or prevent the further oxidation of cracks and pores during the oxidation process. At the same time, this "reinforced concrete" structure of "refractory metal oxide skeleton + flowing b‹o3 glass phase" makes boride ultra-high temperature ceramics excellent in thermal shock resistance. In addition, compared with other ultra-high temperature ceramics (carbide and nitride ultra-high temperature ceramics), boride ultra-high temperature ceramics will form a destructive B 2O ₃ vapor pressure at the interface at a higher temperature (1950 ℃), breaking through the protective layer; The temperature of destructive vapor pressure of CO formed by the oxidation of carbide ultra-high temperature ceramics is 1700 ℃; The temperature at which nitride ceramics produce destructive vapor pressure is lower.
However, the effect of single-phase boride ultra-high temperature ceramics on high temperature oxidation resistance is not ideal, because at temperatures above 1200 ℃, the evaporation rate of glass phase b‹o ₃ is greater than its generation rate, and oxygen directly oxidizes the matrix through the voids of refractory metal oxides. In order to improve its protection temperature range, scientists from all over the world began to dope and modify it to make boride based ultra-high temperature ceramics. The U.S. Air Force Test Department has obtained boride based ultra-high temperature ceramics with good high-temperature oxidation resistance by doping 20% SiC into HfB2 ceramic materials. The U.S. carbondum company has developed a ZrB Ψ + 10%mosi Ψ composite with better oxidation resistance, which is named "border-z" material. This material shows excellent oxidation resistance under the oxidation environment of 1950 ℃.
IV Sintering Densification of boride based ultra high temperature ceramics
The Sintering Densification methods of boride based ultra-high temperature ceramics mainly include pressureless sintering, hot pressing sintering (HP), reaction sintering and discharge plasma sintering (SPS).
1. hot pressing sintering method
Because boride ultra-high temperature ceramics have strong covalent bonds and low self diffusivity, they must be sintered under high temperature and high pressure. In the early research, it was believed that pure boride UHT ceramics could be sintered and densified only at high temperature and high pressure above 2000 ℃ and 20-30Mpa. For example, the original average particle size is 10 μ HfB2 of M was sintered by hot pressing at 2160 ℃ and 27.3mpa for 180min, and the density was less than 95%. Later, it was found that the sintering temperature and pressure could also be reduced to a certain extent by reducing the particle size of raw materials. It is reported that when the average particle size of ZrB2 decreases to 2 μ M, and sintered at 1900 ℃ and 32 MPa for 45 min, a completely dense boride ceramic can be obtained. However, if the average particle size is too small, the sintering will be hindered. Because the particle size is too small, the raw material powder is prone to oxidation, and the formation of oxides will hinder the diffusion and migration of substances in the sintering process.
In the current research literature, there is little about the hot pressing sintering of pure boride, because the sintering temperature can be greatly reduced and the sintering compactness can be improved by adding sintering additives. There are mainly two kinds of additives in the sintering process of boride ultra-high temperature ceramics, one is metal additives, such as Al, Cr, Ni, etc., and the other is ceramic additives based on SiC. It is reported that [14] after adding Ni, ZrB2 can achieve Sintering Densification at 1600 ℃ and 20-50 MPa. In China, hanwenbo [15] and others from Harbin Institute of Technology prepared b4c-zrb2-sic composites by hot pressing sintering method under the sintering temperature of 1900 ℃ and sintering pressure of 30 MPa with B4C as matrix and ZrB2 SiC as additive; Xiezhipeng [16] of Tsinghua University and others prepared (SiC, CNTs) /zrb2 composite ceramics with excellent properties by hot pressing sintering method with silicon, activated carbon and CNTs as additives under the conditions of 1900 ℃ and 30 MPa argon.
2. pressureless sintering
Pressureless sintering is more efficient and economical than hot pressing sintering. Both of them can promote Sintering Densification by adding sintering additives and refining the particle size of raw materials. Previous studies suggested that single-phase pure borides could not be densified in the environment of pressureless sintering, but Baumgartner sintered sub micron TiB2 powder into TiB2 ceramics with a density greater than 99% by pressureless sintering at 2000 ℃ -2100 ℃. Compared with the method of improving the sintering compactness by refining the particle size of raw materials, the method of adding sintering additives is more simple and effective.
In recent studies, under the condition of pressureless sintering, the relative density of ZrB2 ceramics prepared by Kida and Segawa reached more than 95%. However, this kind of Sintering Densification can only be completed by adding BN (5wt.%), AlN (15wt.%), SiC (5wt.%) and other sintering additives at the same time. ZrB2 SiC ceramics were prepared by Pressureless Sintering in Shanghai Silicate Research Institute. Boron powder was used as sintering aid and sintered at 2100 ℃ for 3h. The density of ZrB2 SiC ceramics was 100%; Zhou Changling [20] and others prepared zirconium borate based ZrB2 SiC Composite Ceramics by Pressureless Sintering by adding YAG as sintering additive. The obtained ceramics were uniform, dense and had good mechanical properties.
3. reaction sintering
The principle of reactive sintering is to use the chemical reaction between the original materials to generate a new phase with thermodynamic stability and complete the Sintering Densification at the same time. In this way, the production efficiency can be greatly improved and the cost can be saved, but the reaction process is not easy to control and the grain size is relatively coarse. Some foreign scholars have compared the particle size of ZrB2 ceramics obtained by reactive hot pressing sintering and ordinary hot pressing sintering. The average particle size of 12 μ M, and the average particle size is 6 μ M zrb‹ceramics. Reaction sintering has the advantages of in-situ synthesis and Sintering Densification at the same time. It is used for the sintering preparation of boride based ultra-high temperature composite ceramics ZrB Ψ -sic and HFB Ψ -sic. The reaction formula is as follows:
2Zr + Si + B4C→2ZrB₂ + SiC (12)
(2 + x)Hf + (1 - x)Si + B4C→2HfB₂ + (1 - x)SiC + xHfC (13)
It is worth mentioning that the SiC produced by in-situ reaction sintering can not only greatly reduce the sintering temperature, but also affect the microstructure. The reaction sintering temperature is 1650 ℃, which is lower than the ordinary reaction sintering temperature of 2100 ℃, and the average grain diameter is 2 μ m. Much smaller than the particle size of ordinary reaction sintering 12 μ m。 Wangyujin of Harbin Institute of Technology prepared BN ZrB Ω -zro Ω composites by reaction sintering process. The raw materials such as BN powder, ZrO Э powder, B4C powder, C powder, SiO Э powder and sintering additives were ball milled and sintered according to the composition of the composites. The compactness of the composites reached more than 93%; In addition, zhaiyanxia et al. [25] obtained a bulk density of 2.92g/cm by reaction sintering at 1560 ℃ for 2h in the proportion of b4c/sic=0.6 ³ B4c/sic composite ceramics.
4. discharge plasma sintering (SPS)
Compared with the previous Sintering Densification methods, spark plasma sintering appeared later, but now it has been widely used in the Sintering Densification of various ultra-high temperature ceramic materials. Monteverde et al. Obtained fully dense HfB2 + 30vol.% SiC composite ceramics. Medri et al. Prepared zrb2-zrc-sic composites by HP and SPS at the same time. Without adding sintering additives, HP can only sinter samples with a maximum density of 90% at 1870 ℃, while SPS can obtain fully dense boride composite ceramics at 2100 ℃ for less than 60min.
Zhao Yuan [28] of the Institute of silicate, Chinese Academy of Sciences, etc. used SPS technology to prepare ZrB2 SiC composites with relative density of 98.5% at 1450 ℃ and 30MPa with Zr, B4C and Si powders as starting materials; Huang Anqi of Beijing University of technology and others prepared sic-tib2 multiphase ceramic materials with different compositions at 1700 ℃ and 50MPa by SPS process, using SiC as matrix, TiB2 as second phase and YAG as sintering additive.
V Preparation of boride based ultra high temperature ceramic coating
The main preparation methods of boride based ultra-high temperature ceramic coatings are embedding method, slurry method, vapor deposition method and thermal spraying method.
1. embedding method
The ceramic coating prepared by embedding method was earlier and the technology was more mature. The process is that the matrix sample is placed in the mixed solid powder. Under high temperature conditions, the matrix sample and the solid powder diffuse each other, and then complex physical and chemical reactions occur, so as to form a coating on the surface of the matrix. The embedding mixture consists of four parts: matrix, powder containing coating elements, halides (NaCl, NaF, etc.), and active agents (Al 2O ₃, B 2O ₃, etc.). The process of preparing boride ceramic base coating by embedding method is simple, and the resulting coating is dense and firmly bonded with the matrix. However, the thickness of the coating is difficult to control, and the coating is prone to uneven phenomenon.
Pwang et al. Prepared a layer of ZrB2 sic/sic coating on the graphite surface by embedding method to improve its surface wear resistance and greatly reduce the abrasion rate of the graphite surface. J pourasad[et al. Prepared a layer of sic-zrb2 coating on the surface of SiC modified graphite by embedding method and studied its high-temperature oxidation resistance. The study showed that the oxidation weight gain rate was only 1.1% after 10 hours of oxidation at 1773 K.
Lihejun and others prepared ZrB2 modified silicon-based coating on the surface of carbon / carbon (c/c) composites by embedding method. The prepared coating has a compact structure and has good oxidation resistance at 1773 K, 1873 K and 1953 K. In addition, the embedding method is often used to prepare multi-layer composite ceramic coatings together with other methods. In order to improve the oxidation resistance of c/c composites, Zhang armed [34] and others used the embedding method to prepare the coating SiC transition layer, thermal spraying method to prepare the zrb2-mosi2 outer layer, and prepared the zrb2-mosi2/sic double-layer composite ceramic coating on the c/c composite matrix, which was oxidized for 30h and 10h at 1273K and 1773K respectively, The mass loss of zrb2-mosi2/sic coating samples is 5.3% and 3.0% respectively.
2. slurry method
Preparation of boride based ultra-high temperature ceramic coating by slurry method firstly, boride powder is mixed with binder (varnish, PVB glue, etc.) to form slurry, which is coated on the surface of the substrate, and then sintered in solid or liquid phase under inert gas or vacuum environment to form a coating on the surface of the substrate. Zhang Xiang, et al. Of Central South University prepared zrb‹ based ceramic coatings on the surface of c/c and c/c-sic respectively by slurry method; Wu Dingxing, et al. Combined slurry method and chemical vapor deposition method to prepare SiC (zrb‹ -sic/sic) multi-layer composite anti-oxidation coating. The coating was oxidized at 1500 ℃ for 25h, and the weight of the coating increased by only 2.5%, showing good anti-oxidation property.
3. vapor deposition method
The vapor deposition method is mainly divided into physical vapor deposition (PVD) and chemical vapor deposition (CVD). Both of the two deposition methods can deposit a layer of boride ceramic coating on the surface of the substrate, which is dense, firmly bonded to the substrate and controllable in thickness. The PVD method uses an electron gun to melt and evaporate the ceramic blank under vacuum conditions, and the vapor is deposited on the surface of the substrate to form a coating [38]; The CVD method is to vaporize the raw materials for synthesizing boride ceramics and make them react on the surface of the substrate materials to deposit boride ceramic films.
Boride based ceramic coatings prepared by PVD are generally used on the surfaces of various metal cutting tools. Zhangshushen used high power pulsed magnetron sputtering deposition technology (hipims) to deposit CRB Ψ coating on the surface of cemented carbide tools. The coating showed (101) preferred orientation. The phase structure composition was mainly CrB2 and a small amount of Cr. the atomic ratio of b/cr in the coating was 1.76, and the hardness and elastic modulus were 26.9 ± 1.0GPa and 306.7 ± 6.0 GPA respectively; A layer of tib2/dlc multiphase coating was deposited on aisi1095 steel by SDS Cruz by PVD method, which not only overcomes the brittleness of single-phase TiB2 coating; It also overcomes the defect of insufficient adhesion between DLC coating and substrate. M Berger used hybrid PVD technology to combine electron beam evaporation Ti and magnetron sputtering TiB2 to prepare high hardness coatings with certain ductility. Although the ceramic coating prepared by PVD method is uniform and dense, and has good adhesion with the substrate, compared with other coating preparation methods, its deposition efficiency is too low to prepare a thicker coating.
CVD method makes coating directly from raw materials, which is more efficient than PVD method. Sun Caiyun used CVD technology and ticl4-bcl3-h2 as reaction system to prepare TiB2 wear-resistant coatings on low carbon steel and graphite respectively; Y Xiang prepared ZrB2 SiC ultra-high temperature oxidation resistant coating on the surface of C-SiC composites by combining CVD method and slurry method. The prepared coating has excellent oxidation resistance at different oxidation temperatures; A polycrystalline ZrB2 based ceramic coating was deposited on the graphite surface by P Wang at 1200 ℃ using zrcl4-bcl3-h2 as the reaction system.
4. thermal spraying method
Thermal spraying is a promising surface modification method, which has the unique advantages of fast deposition speed and accurate and controllable coating thickness. In recent years, the preparation of ZrB2, TiB2, CrB2 and other boride ceramic coatings has developed rapidly. Thermal spraying can be divided into many types according to different heat sources. At present, explosive spraying, plasma spraying and laser spraying are commonly used to prepare boride ceramic coatings.
Explosive spraying is to mix oxygen and acetylene in a certain proportion and then detonate. The energy released at the moment of explosion makes the powder melt and impact the substrate surface at a high speed to form a coating. Chengxiangyu prepared zr-o-b ceramic coating by electrothermal explosion spraying. The reaction raw materials were Zr and B2O3 powder. The main components of the coating were zro2-zrb2 and a zirconium compound; SX Hou prepared Mo Si Al coating by electrothermal explosion spraying. The obtained coating has uniform and dense structure and high hardness.
The technology of preparing boride based ceramic coating by plasma spraying is relatively mature and stable, and reports about it emerge in endlessly at home and abroad. Cheng Hanchi [48] sprayed and deposited Al2O3-TiB2 composite powder with three cathode axial powder feeding plasma spraying system (axial- Ⅲ) to obtain tib2/al2o3 coating; Wanghaijun prepared mo-30%nicrbsi coating on al-10si alloy substrate by supersonic plasma spraying. The obtained coating has high hardness and good wear resistance; Cagri tekmen prepared tib2-al2o3 coating by in-situ reactive plasma spraying with Al-12Si, B2O3 and TiO2 raw powders; Iozdemir[51] prepared a layer of al-12si/tib2/h-bn composite coating on the surface of aluminum by atmospheric plasma spraying. The coating has good wear resistance.
Laser spraying, also known as laser cladding, rapidly melts the coating powder and melts the micro area on the surface of the substrate at the same time. The coating and the substrate form a solid metallurgical bond. Laser spraying has no restrictions on the coating materials. It is an ideal preparation method of boride ultra-high temperature ceramic coating. Chun G has prepared a layer of ZrB2 Reinforced Ni based composite coating on the surface of pure titanium by laser cladding. As a result, the surface wear resistance and hardness of pure titanium have been significantly improved; T Simsek prepared a layer of ZrB2 coating on the surface of low carbon steel by CO2 laser cladding. The coating was uniform and compact without cracks and holes. However, at present, the laser cladding technology is not mature. Due to the extremely fast heating and cooling speed, the temperature gradient and thermal expansion coefficient of the coating and the substrate are different, which may lead to the formation of microcracks and holes in the coating process and affect the coating quality.
Vi Outlook
As an ultra-high temperature oxidation resistant material with high melting point, boride ultra-high temperature ceramics have broad application prospects in the aerospace field. However, it is still a long way from large-scale industrial production and application. As a structural material, boride ultra-high temperature ceramics have problems such as brittleness and difficulty in sintering and densification. As a coating material, boride ultra-high temperature ceramics, c/c composite matrix and refractory metal matrix have problems such as mismatching of thermal expansion coefficient. Cracks are easy to occur during the use of the coating. The future research directions of boride ultra-high temperature ceramics are:
(1) By doping single-phase boride ultra-high temperature ceramics, the Sintering Densification Technology was optimized.
(2) The boride based ultra-high temperature ceramic coatings with good adhesion, uniformity, continuity, compactness and excellent microstructure and properties were prepared by developing new coating preparation processes and methods.
Statement: the content of this article is from keyvia New Materials Technology Co., Ltd. for sharing only, and does not represent the position of this number. In case of infringement, please contact the editor to delete it. Thank you!

